Independent coding of movement direction and reward prediction by single pallidal neurons
- PMID: 15537873
- PMCID: PMC6730185
- DOI: 10.1523/JNEUROSCI.2583-04.2004
Independent coding of movement direction and reward prediction by single pallidal neurons
Abstract
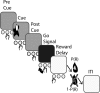
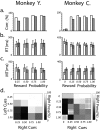
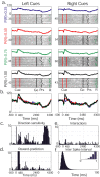
Associating action with its reward value is a basic ability needed by adaptive organisms and requires the convergence of limbic, motor, and associative information. To chart the basal ganglia (BG) involvement in this association, we recorded the activity of 61 well isolated neurons in the external segment of the globus pallidus (GPe) of two monkeys performing a probabilistic visuomotor task. Our results indicate that most (96%) neurons responded to multiple phases of the task. The activity of many (34%) pallidal neurons was modulated solely by direction of movement, and the activity of only a few (3%) pallidal neurons was modulated exclusively by reward prediction. However, the activity of a large number (41%) of single pallidal neurons was comodulated by both expected trial outcome and direction of arm movement. The information carried by the neuronal activity of single pallidal neurons dynamically changed as the trial progressed. The activity was predominantly modulated by both outcome prediction and future movement direction at the beginning of trials and became modulated mainly by movement-direction toward the end of trials. GPe neurons can either increase or decrease their discharge rate in response to predicted future reward. The effects of movement-direction and reward probability on neural activity are linearly summed and thus reflect two independent modulations of pallidal activity. We propose that GPe neurons are uniquely suited for independent processing of a multitude of parameters. This is enabled by the funnel-structure characteristic of the BG architecture, as well as by the anatomical and physiological properties of GPe neurons.
Figures



References
-
- Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366-375. - PubMed
-
- Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266-271. - PubMed
-
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357-381. - PubMed
-
- Bar-Gad I, Morris G, Bergman H (2003) Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol 71: 439-473. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
