Synaptic targeting by Alzheimer's-related amyloid beta oligomers
- PMID: 15537891
- PMCID: PMC6730194
- DOI: 10.1523/JNEUROSCI.3432-04.2004
Synaptic targeting by Alzheimer's-related amyloid beta oligomers
Abstract
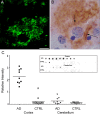
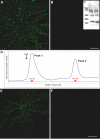
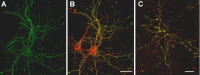
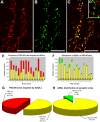
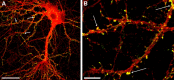
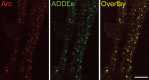
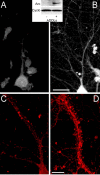
The cognitive hallmark of early Alzheimer's disease (AD) is an extraordinary inability to form new memories. For many years, this dementia was attributed to nerve-cell death induced by deposits of fibrillar amyloid beta (Abeta). A newer hypothesis has emerged, however, in which early memory loss is considered a synapse failure caused by soluble Abeta oligomers. Such oligomers rapidly block long-term potentiation, a classic experimental paradigm for synaptic plasticity, and they are strikingly elevated in AD brain tissue and transgenic-mouse AD models. The current work characterizes the manner in which Abeta oligomers attack neurons. Antibodies raised against synthetic oligomers applied to AD brain sections were found to give diffuse stain around neuronal cell bodies, suggestive of a dendritic pattern, whereas soluble brain extracts showed robust AD-dependent reactivity in dot immunoblots. Antigens in unfractionated AD extracts attached with specificity to cultured rat hippocampal neurons, binding within dendritic arbors at discrete puncta. Crude fractionation showed ligand size to be between 10 and 100 kDa. Synthetic Abeta oligomers of the same size gave identical punctate binding, which was highly selective for particular neurons. Image analysis by confocal double-label immunofluorescence established that >90% of the punctate oligomer binding sites colocalized with the synaptic marker PSD-95 (postsynaptic density protein 95). Synaptic binding was accompanied by ectopic induction of Arc, a synaptic immediate-early gene, the overexpression of which has been linked to dysfunctional learning. Results suggest the hypothesis that targeting and functional disruption of particular synapses by Abeta oligomers may provide a molecular basis for the specific loss of memory function in early AD.
Figures

References
-
- Butterfield DA (2003) Amyloid beta-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer's disease brain: mechanisms and consequences. Curr Med Chem 10: 2651-2659. - PubMed
-
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L (2002) Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid β peptides but not on plaque formation. J Neurosci 22: 10539-10548. - PMC - PubMed
-
- Caughey B, Lansbury Jr PT (2003) Protofibrils, pores, fibrils, and neurode-generation: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26: 267-298. - PubMed
-
- Chang L, Bakhos L, Wang Z, Venton DL, Klein WL (2003) Femtomole immunodetection of synthetic and endogenous amyloid-β oligomers and its application to Alzheimer's disease drug candidate screening. J Mol Neurosci 20: 305-313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
