Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis
- PMID: 15538389
- PMCID: PMC535083
- DOI: 10.1038/sj.emboj.7600418
Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis
Abstract
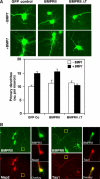
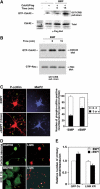
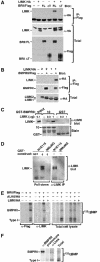
The growth and morphological differentiation of dendrites are critical events in the establishment of proper neuronal connectivity and neural function. One extrinsic factor, BMP7, has been shown to specifically affect dendritic morphogenesis; however, the underlying mechanism by which this occurs is unknown. Here we show that LIM kinase 1 (LIMK1), a key downstream effector of Rho GTPases, colocalizes with the BMP receptor, BMPRII, in the tips of neurites and binds to BMPRII. This interaction is required for BMP-dependent induction of the dendritic arbor in cortical neurons. Furthermore, we demonstrate that the physical interaction of LIMK1 with BMPRII synergizes with the Rho GTPase, Cdc42, to activate LIMK1 catalytic activity. These studies thus define a Smad-independent pathway that directly links the BMP receptor to regulation of actin dynamics and provides insights into how extracellular signals modulate LIMK1 activity to permit fine spatial control over cytoskeletal remodelling during dendritogenesis.
Figures



References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS (2002) wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558 - PubMed
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I (2001) Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4: 367–373 - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393: 805–809 - PubMed
-
- Attisano L, Wrana JL (2002) Signal transduction by the TGF-β superfamily. Science 296: 1646–1647 - PubMed
-
- Bernard V, Bohl BP, Bokock GM (1999) Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem 274: 13198–13204 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

