Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior
- PMID: 15539470
- PMCID: PMC535873
- DOI: 10.1105/tpc.104.026930
Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior
Abstract
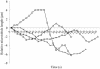
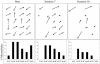
Ordered cortical microtubule arrays are essential for normal plant morphogenesis, but how these arrays form is unclear. The dynamics of individual cortical microtubules are stochastic and cannot fully account for the observed order; however, using tobacco (Nicotiana tabacum) cells expressing either the MBD-DsRed (microtubule binding domain of the mammalian MAP4 fused to the Discosoma sp red fluorescent protein) or YFP-TUA6 (yellow fluorescent protein fused to the Arabidopsis alpha-tubulin 6 isoform) microtubule markers, we identified intermicrotubule interactions that modify their stochastic behaviors. The intermicrotubule interactions occur when the growing plus-ends of cortical microtubules encounter previously existing cortical microtubules. Importantly, the outcome of such encounters depends on the angle at which they occur: steep-angle collisions are characterized by approximately sevenfold shorter microtubule contact times compared with shallow-angle encounters, and steep-angle collisions are twice as likely to result in microtubule depolymerization. Hence, steep-angle collisions promote microtubule destabilization, whereas shallow-angle encounters promote both microtubule stabilization and coalignment. Monte Carlo modeling of the behavior of simulated microtubules, according to the observed behavior of transverse and longitudinally oriented cortical microtubules in cells, reveals that these simple rules for intermicrotubule interactions are necessary and sufficient to facilitate the self-organization of dynamic microtubules into a parallel configuration.
Figures





References
-
- Azimzadeh, J., Traas, J., and Pastuglia, M. (2001). Molecular aspects of microtubule dynamics in plants. Curr. Opin. Plant Biol. 4, 513–519. - PubMed
-
- Baskin, T.I. (2001). On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 215, 150–171. - PubMed
-
- Chan, J., Calder, G.M., Doonan, J.H., and Lloyd, C.W. (2003). EB1 reveals mobile microtubule nucleation sites in Arabidopsis. Nat. Cell Biol. 5, 967–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

