Effect of agmatine on the development of morphine dependence in rats: potential role of cAMP system
- PMID: 15541421
- PMCID: PMC2923207
- DOI: 10.1016/j.ejphar.2004.10.011
Effect of agmatine on the development of morphine dependence in rats: potential role of cAMP system
Abstract
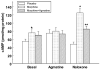
Agmatine is an endogenous amine derived from arginine that potentiates morphine analgesia and blocks symptoms of naloxone-precipitated morphine withdrawal in rats. In this study, we sought to determine whether treatment with agmatine during the development of morphine dependence inhibits the withdrawal symptoms and that the effect is mediated by cAMP system. Exposure of rats to morphine for 7 days resulted in marked naloxone-induced withdrawal symptoms and agmatine treatment along with morphine significantly decreasing the withdrawal symptoms. The levels of cAMP were markedly increased in morphine-treated rat brain slices when incubated with naloxone and this increase was significantly reduced in rats treated with morphine and agmatine. The induction of tyrosine hydroxylase after morphine exposure was also reduced in locus coeruleus when agmatine was administered along with morphine. We conclude that agmatine reduces the development of dependence to morphine and that this effect is probably mediated by the inhibition of cAMP signaling pathway during chronic morphine exposure.
Figures


Similar articles
-
Local opiate withdrawal in locus coeruleus neurons in vitro.J Neurophysiol. 2001 Jun;85(6):2388-97. doi: 10.1152/jn.2001.85.6.2388. J Neurophysiol. 2001. PMID: 11387385
-
Effect of chronic morphine treatment on the biosynthesis of agmatine in rat brain and other tissues.Life Sci. 2002 Aug 23;71(14):1695-701. doi: 10.1016/s0024-3205(02)01911-2. Life Sci. 2002. PMID: 12137915
-
Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal.J Pharmacol Exp Ther. 1999 Aug;290(2):881-92. J Pharmacol Exp Ther. 1999. PMID: 10411605
-
Effects of intragastric agmatine on morphine-induced physiological dependence in beagle dogs and rhesus monkeys.Eur J Pharmacol. 2008 Jun 10;587(1-3):155-62. doi: 10.1016/j.ejphar.2008.03.022. Epub 2008 Mar 29. Eur J Pharmacol. 2008. PMID: 18455724
-
Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats.Life Sci. 1997;61(18):1775-81. doi: 10.1016/s0024-3205(97)00801-1. Life Sci. 1997. PMID: 9365224
Cited by
-
Agmatine: biological role and therapeutic potentials in morphine analgesia and dependence.AAPS J. 2006 Jul 21;8(3):E479-84. doi: 10.1208/aapsj080356. AAPS J. 2006. PMID: 17025265 Free PMC article. Review.
-
Effects of Venlafaxine & Methadone Alone and in Combination with Spontaneous Morphine withdrawal Syndrome & Pain Sensation in Rats.Basic Clin Neurosci. 2015 Jan;6(1):21-8. Basic Clin Neurosci. 2015. PMID: 27504153 Free PMC article.
-
Expression and localization of an agmatinase-like protein in the rat brain.Histochem Cell Biol. 2010 Aug;134(2):137-44. doi: 10.1007/s00418-010-0720-z. Epub 2010 Jul 6. Histochem Cell Biol. 2010. PMID: 20607275
-
Evidence of Reduced Agmatine Concentrations in the Cerebral Cortex of Suicides.Int J Neuropsychopharmacol. 2018 Oct 1;21(10):895-900. doi: 10.1093/ijnp/pyy058. Int J Neuropsychopharmacol. 2018. PMID: 29986038 Free PMC article.
-
Agmatine : metabolic pathway and spectrum of activity in brain.CNS Drugs. 2007;21(11):885-900. doi: 10.2165/00023210-200721110-00002. CNS Drugs. 2007. PMID: 17927294 Review.
References
-
- Aricioglu F, Ercil E, Dulger G. Agmatine inhibits naloxone-induced contractions in morphine-dependent guinea pig ileum. Ann NY Acad Sci. 2003a;1009:147–151. - PubMed
-
- Aricioglu F, Korcegez E, Bozkurt A, Ozyalcin S. Effect of agmatine on acute and mononeuropathic pain. Ann NY Acad Sci. 2003b;1009:106–115. - PubMed
-
- Aricioglu F, Paul IA, Regunathan S. Agmatine reduces only peripheral-related behavioral signs, not the central signs, of morphine withdrawal in nNOS deficient transgenic mice. Neurosci Lett. 2004;354:153–157. - PubMed
-
- Aricioglu-Kartal F, Uzbay IT. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome. Life Sci. 1997;61:1775–1781. - PubMed
-
- Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylylcyclase supersensitization in mu-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem. 1995;270:29732–29738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

