Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut
- PMID: 15542513
- PMCID: PMC1774329
- DOI: 10.1136/gut.2003.034868
Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut
Abstract
Background and aims: The cellular and molecular events involved in ischaemia reperfusion (IR) injury are complex and not fully understood. Previous studies have implicated polymorphonuclear neutrophils (PMN) as major inflammatory cells in IR injury. However, anti-PMN antiserum treatment offers only limited protection, indicating that other inflammatory cells are involved. We have therefore investigated the contribution of resident macrophages in IR injury using an IR gut injury model.
Methods: DA rats were divided into sham operation and IR groups. The superior mesenteric artery was clamped for 30, 45, or 60 minutes (ischaemia) followed by 60 minutes of reperfusion. IR injuries were evaluated by histological staining. Expression of early growth response factor 1 (Egr-1), myeloperoxidase (MPO), and proinflammatory cytokines was analysed by immunohistochemistry, reverse transcription-polymerase chain reaction, and western blotting analysis. The specific role of macrophages in IR gut injury was also evaluated in resident macrophage depleted rats.
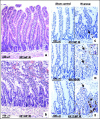
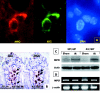
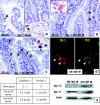
Results: Mucosal sloughing and villi destruction were seen in 45/60 minute and 60/60 minute IR guts. PMN infiltration at the damaged mucosal area was undetectable in 45/60 minute and 60/60 minute IR guts. PMN were localised around the capillaries at the base of the crypts in 60/60 minute IR gut. Obvious PMN infiltration was only observed in damaged villi after three hours of reperfusion. Elevated nuclear Egr-1 immunostaining was localised in resident macrophages at the damaged villi before histological appearance of mucosal damage. Furthermore, resident macrophages at the damaged site expressed MPO. Protein levels of the proinflammatory cytokines RANTES and MCP-1 were increased in IR gut. Depletion of resident macrophages by dichloromethylene bisphosphonate significantly reduced mucosal damage in rat guts after IR.
Conclusion: Our findings indicate that resident macrophages play a role in early mucosal damage in IR gut injury. Therefore, macrophages should be treated as a prime target for therapeutic intervention for IR damage.
Figures



References
-
- Jrvinen O , Laurikka J, Salenius JP, et al. Acute intestinal ischaemia. A review of 214 cases. Ann Chir Gynaecol 1994;83:22–5. - PubMed
-
- Kong SE, Blennerhassett LR, Heel KA, et al. Ischaemia-reperfusion injury to the intestine. Aust N Z J Surg 1998;68:554–61. - PubMed
-
- Jaeschke H . Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg 1998;5:402–8. - PubMed
-
- Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res 1999;43:860–78. - PubMed
-
- Turnage RH, Guice KS, Oldham KT. Pulmonary microvascular injury following intestinal reperfusion. New Horiz 1994;2:463–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
