Effects of folylpolyglutamate synthetase modulation on chemosensitivity of colon cancer cells to 5-fluorouracil and methotrexate
- PMID: 15542523
- PMCID: PMC1774322
- DOI: 10.1136/gut.2004.042713
Effects of folylpolyglutamate synthetase modulation on chemosensitivity of colon cancer cells to 5-fluorouracil and methotrexate
Abstract
Background: Folylpoly-gamma-glutamate synthetase (FPGS) converts intracellular folates and antifolates (for example, methotrexate (MTX)) to polyglutamates. Polyglutamylated folates and antifolates are retained in cells longer and are better substrates than their monoglutamate counterparts for enzymes involved in one carbon transfer. Polyglutamylation of intracellular 5,10-methylenetetrahydrofolate may also enhance the cytotoxicity of 5-fluorouracil (5-FU) by allowing more efficient formation and stabilisation of the inhibitory ternary complex involving thymidylate synthase and a 5-FU metabolite.
Aim: We investigated the effects of FPGS modulation on the chemosensitivity of colon cancer cells to 5-FU and MTX.
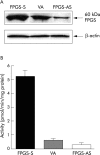
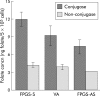
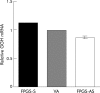
Methods: Human HCT116 colon cancer cells were stably transfected with the sense or antisense FPGS cDNA or blank (control). FPGS protein expression and enzyme activity, growth rate, intracellular folate content and composition, and in vitro chemosensitivity to 5-FU and MTX were determined.
Results: Compared with cells expressing endogenous FPGS, those overexpressing FPGS had significantly faster growth rates and higher concentrations of total folate and long chain folate polyglutamates while antisense FPGS inhibition produced opposite results. FPGS overexpression significantly enhanced, whereas FPGS inhibition decreased, chemosensitivity to 5-FU. No significant difference in chemosensitivity to MTX was observed.
Conclusions: These data provide functional evidence that FPGS overexpression and inhibition modulate chemosensitivity of colon cancer cells to 5-FU by altering intracellular folate polyglutamylation, providing proof of principle. Thus FPGS status may be an important predictor of chemosensitivity of colon cancer cells to 5-FU based chemotherapy, and FPGS gene transfer may increase the sensitivity of colon cancer cells to 5-FU-based chemotherapy.
Figures
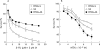
Similar articles
-
Effects of folate and folylpolyglutamyl synthase modulation on chemosensitivity of breast cancer cells.Mol Cancer Ther. 2007 Nov;6(11):2909-20. doi: 10.1158/1535-7163.MCT-07-0449. Mol Cancer Ther. 2007. PMID: 18025275
-
Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines.Clin Cancer Res. 1999 Mar;5(3):643-54. Clin Cancer Res. 1999. PMID: 10100718
-
Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate.J Natl Cancer Inst. 2004 Jan 21;96(2):134-44. doi: 10.1093/jnci/djh015. J Natl Cancer Inst. 2004. PMID: 14734703
-
Enzymes involved in folate metabolism and its implication for cancer treatment.Nutr Res Pract. 2020 Apr;14(2):95-101. doi: 10.4162/nrp.2020.14.2.95. Epub 2020 Mar 6. Nutr Res Pract. 2020. PMID: 32256983 Free PMC article. Review.
-
Folylpoly-γ-glutamate synthetase: A key determinant of folate homeostasis and antifolate resistance in cancer.Drug Resist Updat. 2016 Sep;28:43-64. doi: 10.1016/j.drup.2016.06.004. Epub 2016 Jul 4. Drug Resist Updat. 2016. PMID: 27620954 Review.
Cited by
-
Cytotoxic effects of pemetrexed in gastric cancer cells.Cancer Sci. 2005 Jun;96(6):365-71. doi: 10.1111/j.1349-7006.2005.00058.x. Cancer Sci. 2005. PMID: 15958060 Free PMC article.
-
Histone deacetylase inhibition enhances the therapeutic effects of methotrexate on primary central nervous system lymphoma.Neurooncol Adv. 2020 Jul 3;2(1):vdaa084. doi: 10.1093/noajnl/vdaa084. eCollection 2020 Jan-Dec. Neurooncol Adv. 2020. PMID: 32793886 Free PMC article.
-
Simultaneous Analysis of Wnt and NF-κB Signaling Pathways in Doxorubicin Sensitive and Methotrexate Resistant PLC/ PRF/5 Cells.Cell J. 2016 Winter;17(4):730-9. doi: 10.22074/cellj.2016.3845. Epub 2016 Jan 17. Cell J. 2016. PMID: 26862532 Free PMC article.
-
Intratumoral gene expression of dihydrofolate reductase and folylpoly-c-glutamate synthetase affects the sensitivity to 5-fluorouracil in non-small cell lung cancer.Discov Oncol. 2021 Jun 25;12(1):19. doi: 10.1007/s12672-021-00413-w. Discov Oncol. 2021. PMID: 35201464 Free PMC article.
-
Predicting clinical outcome of 5-fluorouracil-based chemotherapy for colon cancer patients: is the CpG island methylator phenotype the 5-fluorouracil-responsive subgroup?Int J Clin Oncol. 2008 Dec;13(6):498-503. doi: 10.1007/s10147-008-0854-3. Epub 2008 Dec 18. Int J Clin Oncol. 2008. PMID: 19093176 Review.
References
-
- Shane B . Folate chemistry and metabolism. In: Bailey LB, ed. Folate in health and disease. New York: Marcel Dekker, 1995:1–22.
-
- Moran RG. Roles of folylpoly-gamma-glutamate synthetase in therapeutics with tetrahydrofolate antimetabolites: an overview. Semin Oncol 1999;26:24–32. - PubMed
-
- Kamen B . Folate and antifolate pharmacology. Semin Oncol 1997;24:S18–39. - PubMed
-
- Balinska M , Galivan J, Coward JK. Efflux of methotrexate and its polyglutamate derivatives from hepatic cells in vitro. Cancer Res 1981;41:2751–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
