Requirement of Sur2 for efficient replication of mouse adenovirus type 1
- PMID: 15542641
- PMCID: PMC525005
- DOI: 10.1128/JVI.78.23.12888-12900.2004
Requirement of Sur2 for efficient replication of mouse adenovirus type 1
Abstract
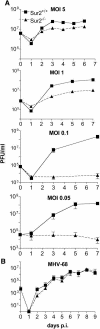
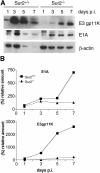
Mouse adenovirus type 1 (MAV-1) early region 1A (E1A) encodes a virulence gene in viral infection of mice. To broaden our understanding of the functions of E1A in MAV-1 pathogenesis, an unbiased experimental approach, glutathione S-transferase (GST) pulldown, was used to screen for cellular proteins that interact with E1A protein. We identified mouse Sur2, a subunit of Mediator complex, as a protein that binds to MAV-1 E1A. The interaction between Sur2 and MAV-1 E1A was confirmed in virus-infected cells. Conserved region 3 (CR3) of MAV-1 E1A was mapped as the region required for Sur2-E1A interaction, as is the case for human adenovirus E1A. Although it has been proposed that human adenovirus E1A recruits the Mediator complex to transactivate transcription of viral early genes, Sur2 function in adenovirus replication has not been directly tested previously. Studies on the functions of Sur2 with mouse embryonic fibroblasts (MEFs) showed that there was a multiplicity-dependent growth defect of MAV-1 in Sur2(-/-) MEFs compared to Sur2(+/+) MEFs. Comparison of the viral DNA and viral mRNA levels in Sur2(+/+) and Sur2(-/-) MEFs confirmed that Sur2 was important for efficient viral replication. The viral replication defects in Sur2(-/-) MEFs appeared to be due at least in part to a defect in viral early gene transcription.
Figures


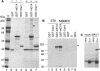



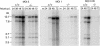


Similar articles
-
E1A-CR3 interaction-dependent and -independent functions of mSur2 in viral replication of early region 1A mutants of mouse adenovirus type 1.J Virol. 2005 Mar;79(6):3267-76. doi: 10.1128/JVI.79.6.3267-3276.2005. J Virol. 2005. PMID: 15731221 Free PMC article.
-
Interaction of mouse adenovirus type 1 early region 1A protein with cellular proteins pRb and p107.Virology. 1996 Oct 1;224(1):184-97. doi: 10.1006/viro.1996.0520. Virology. 1996. PMID: 8862413
-
Contributions of E1A to mouse adenovirus type 1 pathogenesis following intranasal inoculation.Virology. 2007 Jan 5;357(1):54-67. doi: 10.1016/j.virol.2006.08.013. Epub 2006 Sep 7. Virology. 2007. PMID: 16962154 Free PMC article.
-
Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus.Oncogene. 2005 Nov 21;24(52):7673-85. doi: 10.1038/sj.onc.1209040. Oncogene. 2005. PMID: 16299528 Review.
-
Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes.J Virol. 2008 Aug;82(15):7252-63. doi: 10.1128/JVI.00104-08. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385237 Free PMC article. Review. No abstract available.
Cited by
-
Mouse Adenovirus Type 1 Early Region 1A Effects on the Blood-Brain Barrier.mSphere. 2016 Apr 20;1(2):e00079-16. doi: 10.1128/mSphere.00079-16. eCollection 2016 Mar-Apr. mSphere. 2016. PMID: 27303733 Free PMC article.
-
Acute respiratory infection with mouse adenovirus type 1.Virology. 2005 Sep 30;340(2):245-54. doi: 10.1016/j.virol.2005.06.021. Virology. 2005. PMID: 16054189 Free PMC article.
-
Comparison of E1A CR3-dependent transcriptional activation across six different human adenovirus subgroups.J Virol. 2010 Dec;84(24):12771-81. doi: 10.1128/JVI.01243-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881041 Free PMC article.
-
Role of mouse adenovirus type 1 E4orf6-induced degradation of protein kinase R in pathogenesis.J Virol. 2025 Feb 25;99(2):e0154524. doi: 10.1128/jvi.01545-24. Epub 2024 Dec 31. J Virol. 2025. PMID: 39745442 Free PMC article.
-
E1A-CR3 interaction-dependent and -independent functions of mSur2 in viral replication of early region 1A mutants of mouse adenovirus type 1.J Virol. 2005 Mar;79(6):3267-76. doi: 10.1128/JVI.79.6.3267-3276.2005. J Virol. 2005. PMID: 15731221 Free PMC article.
References
-
- Ball, A. O., C. W. Beard, S. D. Redick, and K. R. Spindler. 1989. Genome organization of mouse adenovirus type 1 early region 1: A novel transcription map. Virology 170:523-536. - PubMed
-
- Ball, A. O., C. W. Beard, P. Villegas, and K. R. Spindler. 1991. Early region 4 sequence and biological comparison of two isolates of mouse adenovirus type 1. Virology 180:257-265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

