Two modes of pseudorabies virus neuroinvasion and lethality in mice
- PMID: 15542647
- PMCID: PMC525033
- DOI: 10.1128/JVI.78.23.12951-12963.2004
Two modes of pseudorabies virus neuroinvasion and lethality in mice
Abstract
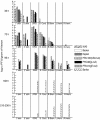
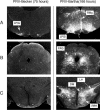
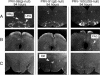
We describe two distinct modes of neuroinvasion and lethality after murine flank inoculation with virulent and attenuated strains of pseudorabies virus (PRV). Mice infected with virulent (e.g., PRV-Becker, PRV-Kaplan, or PRV-NIA3) strains self-mutilate their flank skin in response to virally induced pruritus, die rapidly with no identifiable symptoms of central nervous system (CNS) infection such as behavioral abnormalities, and have little infectious virus or viral antigen in the brain. In distinct contrast, animals infected with an attenuated PRV vaccine strain (PRV-Bartha) survive approximately three times longer than wild-type PRV-infected animals, exhibit severe CNS abnormalities, and have an abundance of infectious virus in the brain at the time of death. Interestingly, these animals have no skin lesions and do not appear pruritic at any time during infection. The severe pruritus and relatively earlier time until death induced by wild-type PRV infection may reflect the peripheral nervous system (PNS) and immune responses to infection rather than a fatal, virally induced CNS pathology. Based on previously characterized afferent (sensory) and efferent (motor) neuronal pathways that innervate the skin, we deduced that wild-type virulent strains transit through the PNS via both afferent and efferent routes, whereas PRV-Bartha travels by only efferent routes in the PNS en route to the brain.
Figures






References
-
- Babic, N., B. Klupp, A. Brack, T. C. Mettenleiter, G. Ugolini, and A. Flamand. 1996. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology 219:279-284. - PubMed
-
- Bartha, A. 1961. Experimental reduction of virulence of Aujeszky's disease virus. Mag. Allat. Lap. 16:42-45.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

