Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1
- PMID: 15542649
- PMCID: PMC525028
- DOI: 10.1128/JVI.78.23.12975-12986.2004
Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1
Abstract
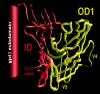
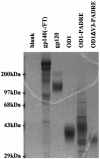
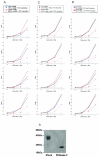
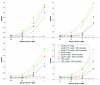
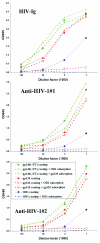
The core of the gp120 glycoprotein from human immunodeficiency virus type 1 (HIV-1) is comprised of three major structural domains: the outer domain, the inner domain, and the bridging sheet. The outer domain is exposed on the HIV-1 envelope glycoprotein trimer and contains binding surfaces for neutralizing antibodies such as 2G12, immunoglobulin G1b12, and anti-V3 antibodies. We expressed the outer domain of HIV-1(YU2) gp120 as an independent protein, termed OD1. OD1 efficiently bound 2G12 and a large number of anti-V3 antibodies, indicating its structural integrity. Immunochemical studies with OD1 indicated that antibody responses against the outer domain of the HIV-1 gp120 envelope glycoprotein are rare in HIV-1-infected human sera that potently neutralize the virus. Surprisingly, such outer-domain-directed antibody responses are commonly elicited by immunization with recombinant monomeric gp120. Immunization with soluble, stabilized HIV-1 envelope glycoprotein trimers elicited antibody responses that more closely resembled those in the sera of HIV-1-infected individuals. These results underscore the qualitatively different humoral immune responses elicited during natural infection and after gp120 vaccination and help to explain the failure of gp120 as an effective vaccine.
Figures



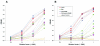
Similar articles
-
Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants.J Virol. 2006 Feb;80(3):1414-26. doi: 10.1128/JVI.80.3.1414-1426.2006. J Virol. 2006. PMID: 16415019 Free PMC article.
-
Comparison of HIV Type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies.AIDS Res Hum Retroviruses. 2005 Jan;21(1):58-67. doi: 10.1089/aid.2005.21.58. AIDS Res Hum Retroviruses. 2005. PMID: 15665645
-
Neutralizing antibodies from the sera of human immunodeficiency virus type 1-infected individuals bind to monomeric gp120 and oligomeric gp140.J Virol. 1998 Dec;72(12):9656-67. doi: 10.1128/JVI.72.12.9656-9667.1998. J Virol. 1998. PMID: 9811699 Free PMC article.
-
GP120: target for neutralizing HIV-1 antibodies.Annu Rev Immunol. 2006;24:739-69. doi: 10.1146/annurev.immunol.24.021605.090557. Annu Rev Immunol. 2006. PMID: 16551265 Review.
-
Human immunodeficiency virus.Biotechnology. 1992;20:309-26. doi: 10.1016/b978-0-7506-9265-6.50019-1. Biotechnology. 1992. PMID: 1600383 Review.
Cited by
-
Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS.Retrovirology. 2007 Dec 12;4:89. doi: 10.1186/1742-4690-4-89. Retrovirology. 2007. PMID: 18076768 Free PMC article.
-
Temsavir blocks the immunomodulatory activities of HIV-1 soluble gp120.Cell Chem Biol. 2023 May 18;30(5):540-552.e6. doi: 10.1016/j.chembiol.2023.03.003. Epub 2023 Mar 22. Cell Chem Biol. 2023. PMID: 36958337 Free PMC article.
-
The selection of low envelope glycoprotein reactivity to soluble CD4 and cold during simian-human immunodeficiency virus infection of rhesus macaques.J Virol. 2014 Jan;88(1):21-40. doi: 10.1128/JVI.01558-13. Epub 2013 Oct 16. J Virol. 2014. PMID: 24131720 Free PMC article.
-
Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C.J Virol. 2008 Dec;82(23):11651-68. doi: 10.1128/JVI.01762-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815292 Free PMC article.
-
Mapping the immune response to the outer domain of a human immunodeficiency virus-1 clade C gp120.J Gen Virol. 2008 Oct;89(Pt 10):2597-2604. doi: 10.1099/vir.0.2008/003491-0. J Gen Virol. 2008. PMID: 18796729 Free PMC article.
References
-
- Alexander, J., J. Sidney, S. Southwood, J. Ruppert, C. Oseroff, A. Maewal, K. Snoke, H. M. Serra, R. T. Kubo, A. Sette, et al. 1994. Development of high potency universal DR-restricted helper epitopes by modification of high-affinity DR-blocking peptides. Immunity 1:751-761. - PubMed
-
- Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091-1094. - PubMed
-
- Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. - PubMed
-
- Beddows, S., S. Louisirirotchanakul, R. Cheingsong-Popov, P. J. Easterbrook, P. Simmonds, and J. Weber. 1998. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J. Gen. Virol. 79:77-82. - PubMed
-
- Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, et al. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

