A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity
- PMID: 15542851
- PMCID: PMC529046
- DOI: 10.1128/MCB.24.23.10437-10447.2004
A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity
Abstract
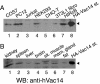
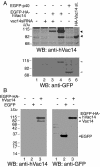
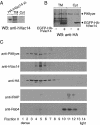
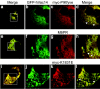
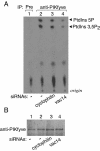
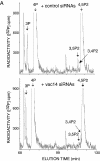
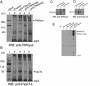
Multivesicular body morphology and size are controlled in part by PtdIns(3,5)P(2), produced in mammalian cells by PIKfyve-directed phosphorylation of PtdIns(3)P. Here we identify human Vac14 (hVac14), an evolutionarily conserved protein, present in all eukaryotes but studied principally in yeast thus far, as a novel positive regulator of PIKfyve enzymatic activity. In mammalian cells and tissues, Vac14 is a low-abundance 82-kDa protein, but its endogenous levels could be up-regulated upon ectopic expression of hVac14. PIKfyve and hVac14 largely cofractionated, populated similar intracellular locales, and physically associated. A small-interfering RNA-directed gene-silencing approach to selectively eliminate endogenous hVac14 rendered HEK293 cells susceptible to morphological alterations similar to those observed upon expression of PIKfyve mutants deficient in PtdIns(3,5)P(2) production. Largely decreased in vitro PIKfyve kinase activity and unaltered PIKfyve protein levels were detected under these conditions. Conversely, ectopic expression of hVac14 increased the intrinsic PIKfyve lipid kinase activity. Concordantly, intracellular PtdIns(3)P-to-PtdIns(3,5)P(2) conversion was perturbed by hVac14 depletion and was elevated upon ectopic expression of hVac14. These data demonstrate a major role of the PIKfyve-associated hVac14 protein in activating PIKfyve and thereby regulating PtdIns(3,5)P(2) synthesis and endomembrane homeostasis in mammalian cells.
Figures
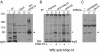




Similar articles
-
Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway.J Biol Chem. 2005 Mar 4;280(9):7883-9. doi: 10.1074/jbc.M412729200. Epub 2004 Nov 16. J Biol Chem. 2005. PMID: 15546865
-
Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex.J Biol Chem. 2007 Aug 17;282(33):23878-91. doi: 10.1074/jbc.M611678200. Epub 2007 Jun 7. J Biol Chem. 2007. PMID: 17556371
-
Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles.J Cell Sci. 2004 Dec 1;117(Pt 25):5985-93. doi: 10.1242/jcs.01517. Epub 2004 Nov 16. J Cell Sci. 2004. PMID: 15546921
-
The amyloid precursor protein (APP) binds the PIKfyve complex and modulates its function.Biochem Soc Trans. 2016 Feb;44(1):185-90. doi: 10.1042/BST20150179. Biochem Soc Trans. 2016. PMID: 26862204 Review.
-
Roles of PIKfyve in multiple cellular pathways.Curr Opin Cell Biol. 2022 Jun;76:102086. doi: 10.1016/j.ceb.2022.102086. Epub 2022 May 16. Curr Opin Cell Biol. 2022. PMID: 35584589 Free PMC article. Review.
Cited by
-
The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve-/- embryos but normality of PIKfyve+/- mice.J Biol Chem. 2011 Apr 15;286(15):13404-13. doi: 10.1074/jbc.M111.222364. Epub 2011 Feb 24. J Biol Chem. 2011. PMID: 21349843 Free PMC article.
-
Distinct Requirements for Vacuolar Protein Sorting 34 Downstream Effector Phosphatidylinositol 3-Phosphate 5-Kinase in Podocytes Versus Proximal Tubular Cells.J Am Soc Nephrol. 2016 Sep;27(9):2702-19. doi: 10.1681/ASN.2015050555. Epub 2016 Jan 29. J Am Soc Nephrol. 2016. PMID: 26825532 Free PMC article.
-
Phosphoinositides in the mammalian endo-lysosomal network.Subcell Biochem. 2012;59:65-110. doi: 10.1007/978-94-007-3015-1_3. Subcell Biochem. 2012. PMID: 22374088 Free PMC article. Review.
-
The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover.J Cell Biol. 2006 Feb 27;172(5):693-704. doi: 10.1083/jcb.200512105. Epub 2006 Feb 21. J Cell Biol. 2006. PMID: 16492811 Free PMC article.
-
Apilimod, a candidate anticancer therapeutic, arrests not only PtdIns(3,5)P2 but also PtdIns5P synthesis by PIKfyve and induces bafilomycin A1-reversible aberrant endomembrane dilation.PLoS One. 2018 Sep 21;13(9):e0204532. doi: 10.1371/journal.pone.0204532. eCollection 2018. PLoS One. 2018. PMID: 30240452 Free PMC article.
References
-
- Dove, S. K., F. T. Cooke, M. R. Douglas, L. G. Sayers, P. J. Parker, and R. H. Michell. 1997. Osmotic stress activates phosphatidylinositol-3,5-bisphospahte synthesis. Nature 390:187-192. - PubMed
-
- Dove, S. K., R. K. McEwen, A. Mayes, D. C. Hughes, J. D. Beggs, and R. H. Michell. 2002. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr. Biol. 12:885-893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
