Yeast shuttling SR proteins Npl3p, Gbp2p, and Hrb1p are part of the translating mRNPs, and Npl3p can function as a translational repressor
- PMID: 15542855
- PMCID: PMC529038
- DOI: 10.1128/MCB.24.23.10479-10491.2004
Yeast shuttling SR proteins Npl3p, Gbp2p, and Hrb1p are part of the translating mRNPs, and Npl3p can function as a translational repressor
Abstract
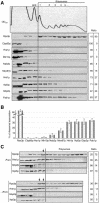
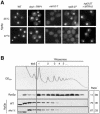
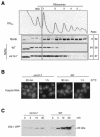
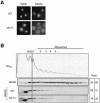
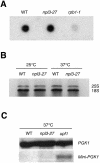
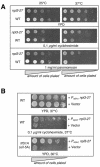
A major challenge in current molecular biology is to understand how sequential steps in gene expression are coupled. Recently, much attention has been focused on the linkage of transcription, processing, and mRNA export. Here we describe the cytoplasmic rearrangement for shuttling mRNA binding proteins in Saccharomyces cerevisiae during translation. While the bulk of Hrp1p, Nab2p, or Mex67p is not associated with polysome containing mRNAs, significant amounts of the serine/arginine (SR)-type shuttling mRNA binding proteins Npl3p, Gbp2p, and Hrb1p remain associated with the mRNA-protein complex during translation. Interestingly, a prolonged association of Npl3p with polysome containing mRNAs results in translational defects, indicating that Npl3p can function as a negative translational regulator. Consistent with this idea, a mutation in NPL3 that slows down translation suppresses growth defects caused by the presence of translation inhibitors or a mutation in eIF5A. Moreover, using sucrose density gradient analysis, we provide evidence that the import receptor Mtr10p, but not the SR protein kinase Sky1p, is involved in the timely regulated release of Npl3p from polysome-associated mRNAs. Together, these data shed light onto the transformation of an exporting to a translating mRNP.
Figures


Similar articles
-
Differential export requirements for shuttling serine/arginine-type mRNA-binding proteins.J Biol Chem. 2004 Feb 13;279(7):5049-52. doi: 10.1074/jbc.C300522200. Epub 2003 Dec 15. J Biol Chem. 2004. PMID: 14676199
-
Uncoupling of the hnRNP Npl3p from mRNAs during the stress-induced block in mRNA export.Genes Dev. 1999 Aug 1;13(15):1994-2004. doi: 10.1101/gad.13.15.1994. Genes Dev. 1999. PMID: 10444597 Free PMC article.
-
Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation.RNA. 2001 Feb;7(2):302-13. doi: 10.1017/s1355838201002369. RNA. 2001. PMID: 11233987 Free PMC article.
-
[Nuclear transport of hnRNP and mRNA].Tanpakushitsu Kakusan Koso. 2000 Oct;45(14):2378-87. Tanpakushitsu Kakusan Koso. 2000. PMID: 11051839 Review. Japanese. No abstract available.
-
Nuclear export of mRNA.FEBS Lett. 2001 Jun 8;498(2-3):150-6. doi: 10.1016/s0014-5793(01)02482-6. FEBS Lett. 2001. PMID: 11412847 Review.
Cited by
-
RGG-motif containing mRNA export factor Gbp2 acts as a translation repressor.RNA Biol. 2021 Dec;18(12):2342-2353. doi: 10.1080/15476286.2021.1910403. Epub 2021 Apr 29. RNA Biol. 2021. PMID: 33910495 Free PMC article.
-
Two alternatively spliced isoforms of the Arabidopsis SR45 protein have distinct roles during normal plant development.Plant Physiol. 2009 Jul;150(3):1450-8. doi: 10.1104/pp.109.138180. Epub 2009 Apr 29. Plant Physiol. 2009. PMID: 19403727 Free PMC article.
-
Keeping mRNPs in check during assembly and nuclear export.Nat Rev Mol Cell Biol. 2011 Jun;12(6):377-84. doi: 10.1038/nrm3119. Nat Rev Mol Cell Biol. 2011. PMID: 21602906
-
Surveillance of 3' mRNA cleavage during transcription termination requires CF IB/Hrp1.Nucleic Acids Res. 2023 Sep 8;51(16):8758-8773. doi: 10.1093/nar/gkad530. Nucleic Acids Res. 2023. PMID: 37351636 Free PMC article.
-
The shuttling protein Npl3 promotes translation termination accuracy in Saccharomyces cerevisiae.J Mol Biol. 2009 Dec 4;394(3):410-22. doi: 10.1016/j.jmb.2009.08.067. Epub 2009 Sep 3. J Mol Biol. 2009. PMID: 19733178 Free PMC article.
References
-
- Afonina, E., R. Stauber, and G. N. Pavlakis. 1998. The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J. Biol. Chem. 273:13015-13021. - PubMed
-
- Benne, R., M. L. Brown-Luedi, and J. W. Hershey. 1978. Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J. Biol. Chem. 253:3070-3077. - PubMed
-
- Dreyfuss, G., V. N. Kim, and N. Kataoka. 2002. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 3:195-205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
