Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model
- PMID: 15544612
- PMCID: PMC1809236
- DOI: 10.1111/j.1365-2249.2004.02649.x
Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model
Abstract
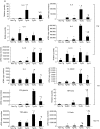
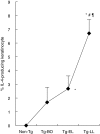
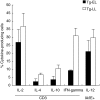
We investigated cytokine profiles in interleukin (IL)-4 transgenic (Tg) mice with a skin inflammatory disease resembling human atopic dermatitis. cDNA microarray revealed that the mRNAs encoding IL-1beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-13, tumour necrosis factor (TNF)-alpha, TNF-beta and interferon (IFN)-gamma were up-regulated in the skin of late lesion Tg mice and to a lesser degree in non-lesion Tg mice when compared to those of non-Tg mice. Real time reverse transcription-polymerase chain reaction (RT-PCR) analyses indicated that the cDNA copy numbers of IL-1beta, IL-4, IL-6, IL-10, TNF-alpha and IFN-gamma from the skin of late, early and non-lesions increased significantly compared to non-Tg mice. IL-2 and IL-12p40 cDNA copy numbers were increased significantly in early, but not late, lesions. Interestingly, IL-1beta, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-alpha, and IFN-gamma cDNAs were increased significantly the skin of before-onset and/or non-lesion mice. Flow cytometry analyses demonstrated an increased percentage of keratinocytes producing IL-4 as the disease progressed. The percentage of IL-2, IL-4, IL-10 and IFN-gamma-producing T cells and IL-12-producing antigen-presenting cells in skin-draining lymph nodes and inflammatory skin also increased, particularly in mice with late lesion. These results suggest that disease induction is primarily triggered by Th2 cytokines and that Th1, Th2 and non-Th proinflammatory cytokines are all involved in the disease process.
Figures



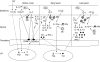
 : migration, LC: Langerhans cells; DC, dendritic cells; KC, keratinocytes; MC, mast cells; Mφ, macrophages; •: Th1 lymphocytes; ○: Th2 lymphocytes; LN, skin-draining lymph node.
: migration, LC: Langerhans cells; DC, dendritic cells; KC, keratinocytes; MC, mast cells; Mφ, macrophages; •: Th1 lymphocytes; ○: Th2 lymphocytes; LN, skin-draining lymph node.References
-
- Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–60. - PubMed
-
- Leung DYM, Jain N, Leo HL. New concepts in the pathogenesis of atopic dermatitis. Curr Opin Immunol. 2003;15:634–8. - PubMed
-
- Hanifin JM. Immunologic aspects of atopic dermatitis. Dermatol Clin. 1990;8:747–50. - PubMed
-
- Cooper KD, Stevens SR. T cells in atopic dermatitis. J Am Acad Dermatol. 2001;45(Suppl. 1):S10–12. - PubMed
-
- Cooper KD. Atopic dermatitis: recent trends in pathogenesis and therapy. J Invest Dermatol. 1994;102:128–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

