Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants
- PMID: 15545317
- PMCID: PMC2172580
- DOI: 10.1083/jcb.200405144
Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants
Abstract
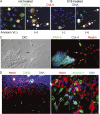
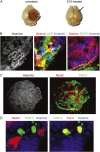
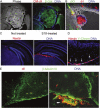
The formation of stem cell-derived tumors (teratomas) is observed when engrafting undifferentiated embryonic stem (ES) cells, embryoid body-derived cells (EBCs), or mammalian embryos and is a significant obstacle to stem cell therapy. We show that in tumors formed after engraftment of EBCs into mouse brain, expression of the pluripotency marker Oct-4 colocalized with that of prostate apoptosis response-4 (PAR-4), a protein mediating ceramide-induced apoptosis during neural differentiation of ES cells. We tested the ability of the novel ceramide analogue N-oleoyl serinol (S18) to eliminate mouse and human Oct-4(+)/PAR-4(+) cells and to increase the proportion of nestin(+) neuroprogenitors in EBC-derived cell cultures and grafts. S18-treated EBCs persisted in the hippocampal area and showed neuronal lineage differentiation as indicated by the expression of beta-tubulin III. However, untreated cells formed numerous teratomas that contained derivatives of endoderm, mesoderm, and ectoderm. Our results show for the first time that ceramide-induced apoptosis eliminates residual, pluripotent EBCs, prevents teratoma formation, and enriches the EBCs for cells that undergo neural differentiation after transplantation.
Figures



References
-
- Barberi, T., P. Klivenyi, N.Y. Calingasan, H. Lee, H. Kawamata, K. Loonam, A.L. Perrier, J. Bruses, M.E. Rubio, N. Topf, et al. 2003. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 21:1200–1207. - PubMed
-
- Bieberich, E., T. Kawaguchi, and R.K. Yu. 2000. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J. Biol. Chem. 275:177–181. - PubMed
-
- Bieberich, E., S. MacKinnon, J. Silva, and R.K. Yu. 2001. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J. Biol. Chem. 276:44396–44404. - PubMed
-
- Bieberich, E., B. Hu, J. Silva, S. MacKinnon, R.K. Yu, H. Fillmore, W.C. Broaddus, and R.M. Ottenbrite. 2002. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 181:55–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

