Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense
- PMID: 15545358
- PMCID: PMC2211915
- DOI: 10.1084/jem.20040437
Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense
Abstract
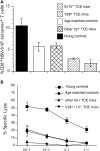
Peripheral T cell diversity is virtually constant in the young, but is invariably reduced in aged mice and humans. CD8+ T cell clonal expansions (TCE) are the most drastic manifestation of, and possible contributors to, this reduced diversity. We show that the presence of TCE results in reduced CD8+, but not CD4+, T cell diversity, and in functional inability to mobilize parts of the CD8+ T cell repertoire affected by TCE. In the model of herpes simplex virus (HSV)-1 infection of B6 mice, >90% of the responding CD8+ T cells use Vbeta10 or Vbeta8 and are directed against a single glycoprotein B (gB498-505) epitope, gB-8p. We found that old animals bearing CD8+ TCE within Vbeta10 or Vbeta8 families failed to mount an effective immune response against HSV-1, as judged by reduced numbers of peptide-major histocompatibility complex tetramer+ CD8 T cells and an absence of antiviral lytic function. Furthermore, Vbeta8 TCE experimentally introduced into young mice resulted in lower resistance to viral challenge, whereas Vbeta5+ TCE induced in a similar fashion did not impact viral resistance. These results demonstrate that age-related TCE functionally impair the efficacy of antiviral CD8+ T cell immunity in an antigen-specific manner, strongly suggesting that TCE are not the mere manifestation of, but are also a contributing factor to, the immunodeficiency of senescence.
Figures





References
-
- Miller, R.A. 1996. The aging immune system: primer and prospectus. Science. 273:70–74. - PubMed
-
- Pawelec, G., M. Adibzadeh, R. Solana, and I. Beckman. 1997. The T cell in the ageing individual. Mech. Ageing Dev. 93:35–45. - PubMed
-
- Ku, C.C., B. Kotzin, J. Kappler, and P. Marrack. 1997. CD8+ T-cell clones in old mice. Immunol. Rev. 160:139–144. - PubMed
-
- Globerson, A., and R.B. Effros. 2000. Ageing of lymphocytes and lymphocytes in the aged. Immunol. Today. 21:515–521. - PubMed
-
- Hingorani, R., I.H. Choi, P. Akolkar, B. Gulwani-Akolkar, R. Pergolizzi, J. Silver, and P.K. Gregersen. 1993. Clonal predominance of T cell receptors within the CD8+ CD45 RO+ subset in normal human subjects. J. Immunol. 151:5762–5769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

