Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori
- PMID: 15545611
- PMCID: PMC528734
- DOI: 10.1073/pnas.0407596101
Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori
Abstract
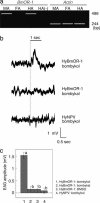
Sex pheromones released by female moths are detected with high specificity and sensitivity in the olfactory sensilla of antennae of conspecific males. Bombykol in the silkmoth Bombyx mori was the first sex pheromone to be identified. Here we identify a male-specific G protein-coupled olfactory receptor gene, B. mori olfactory receptor 1 (BmOR-1), that appears to encode a bombykol receptor. The BmOR-1 gene is located on the Z sex chromosome, has an eight-exon/seven-intron structure, and exhibits male-specific expression in the pheromone receptor neurons of male moth antenna during late pupal and adult stages. Bombykol stimulation of Xenopus laevis oocytes expressing BmOR-1 and BmGalphaq elicited robust dose-dependent inward currents on two-electrode voltage clamp recordings, demonstrating that the binding of bombykol to BmOR-1 leads to the activation of a BmGalphaq-mediated signaling cascade. Antennae of female moths infected with BmOR-1-recombinant baculovirus showed electrophysiological responses to bombykol but not to bombykal. These results provide evidence that BmOR-1 is a G protein-coupled sex pheromone receptor that recognizes bombykol.
Figures


References
-
- Karlson, P. & Lüscher, M. (1959) Nature 183, 55–56. - PubMed
-
- Butenandt, V. A., Beckmann, R., Stamm, D. & Hecker, E. (1959) Z. Naturforsch. 14, 283–284.
-
- Schneider, D. (1969) Science 163, 1031–1037. - PubMed
-
- Kaissling, K.-E. (1987) R. H. Wright Lectures on Insect Olfaction, ed. Colbow, K. (Simon Fraser Univ., Burnaby, Canada).
-
- Kaissling, K.-E. & Kasang, G. (1978) Naturwissenschaften 65, 382–384.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

