LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis
- PMID: 15545629
- PMCID: PMC528889
- DOI: 10.1101/gad.1249504
LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis
Abstract
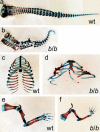
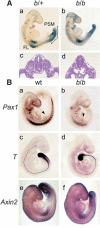
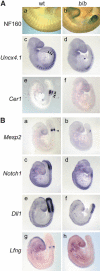
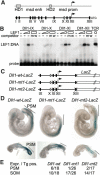
Wnt signaling, which is mediated by LEF1/TCF transcription factors, has been placed upstream of the Notch pathway in vertebrate somitogenesis. Here, we examine the molecular basis for this presumed hierarchy and show that a targeted mutation of Lef1, which abrogates LEF1 function and impairs the activity of coexpressed TCF factors, affects the patterning of somites and the expression of components of the Notch pathway. LEF1 was found to bind multiple sites in the Dll1 promoter in vitro and in vivo. Moreover, mutations of LEF1-binding sites in the Dll1 promoter impair expression of a Dll1-LacZ transgene in the presomitic mesoderm. Finally, the induced expression of LEF1-beta-catenin activates the expression of endogenous Dll1 in fibroblastic cells. Thus, Wnt signaling can affect the Notch pathway by a LEF1-mediated regulation of Dll1.
Figures
Similar articles
-
WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos.Genes Dev. 2004 Nov 15;18(22):2712-7. doi: 10.1101/gad.1248604. Genes Dev. 2004. PMID: 15545628 Free PMC article.
-
Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8358-63. doi: 10.1073/pnas.150152697. Proc Natl Acad Sci U S A. 2000. PMID: 10890911 Free PMC article.
-
Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for Wnt signaling during neural patterning in Xenopus.Mech Dev. 1999 Sep;87(1-2):21-32. doi: 10.1016/s0925-4773(99)00136-7. Mech Dev. 1999. PMID: 10495268
-
Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S31-9. J Bone Joint Surg Am. 2001. PMID: 11263663 Review.
-
Oscillatory control of Delta-like1 in somitogenesis and neurogenesis: A unified model for different oscillatory dynamics.Semin Cell Dev Biol. 2016 Jan;49:76-82. doi: 10.1016/j.semcdb.2016.01.017. Epub 2016 Jan 24. Semin Cell Dev Biol. 2016. PMID: 26818178 Review.
Cited by
-
Wnt3a links left-right determination with segmentation and anteroposterior axis elongation.Development. 2005 Dec;132(24):5425-36. doi: 10.1242/dev.02149. Epub 2005 Nov 16. Development. 2005. PMID: 16291790 Free PMC article.
-
Lymphoid enhancer-binding factor-1 promotes stemness and poor differentiation of hepatocellular carcinoma by directly activating the NOTCH pathway.Oncogene. 2019 May;38(21):4061-4074. doi: 10.1038/s41388-019-0704-y. Epub 2019 Jan 29. Oncogene. 2019. PMID: 30696957
-
Lats2 modulates adipocyte proliferation and differentiation via hippo signaling.PLoS One. 2013 Aug 16;8(8):e72042. doi: 10.1371/journal.pone.0072042. eCollection 2013. PLoS One. 2013. PMID: 23977200 Free PMC article.
-
Sodium ferulate inhibits neointimal hyperplasia in rat balloon injury model.PLoS One. 2014 Jan 29;9(1):e87561. doi: 10.1371/journal.pone.0087561. eCollection 2014. PLoS One. 2014. PMID: 24489938 Free PMC article.
-
Chromatin-based mechanisms of renal epithelial differentiation.J Am Soc Nephrol. 2011 Jul;22(7):1208-12. doi: 10.1681/ASN.2010101018. Epub 2011 Jun 23. J Am Soc Nephrol. 2011. PMID: 21700830 Free PMC article. Review.
References
-
- Adams R.H., Betz, H., and Puschel, A.W. 1996. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech. Dev. 57: 33-45. - PubMed
-
- Aulehla A., Wehrle, C., Brand-Saberi, B., Kemler, R., Gossler, A., Kanzler, B., and Herrmann, B.G. 2003. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 4: 395-406. - PubMed
-
- Beckers J., Caron, A., Hrabé de Angelis, M., Hans, S., Campos-Ortega, J.A., and Gossler, A. 2000. Distinct regulatory elements direct Delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech. Dev. 95: 23-34. - PubMed
-
- Biben C., Stanley, E., Fabri, L., Kotecha, S., Rhinn, M., Drinkwater, C., Lab, M., Wang, C.C. Nash, A., Hilton, D., et al. 1998. Murine cerberus homologue mCer-1: A candidate anterior patterning molecule. Dev. Biol. 194: 135-151. - PubMed
-
- Conlon R.A., Reaume, A.G., and Rossant, J. 1995. Notch1 is required for the coordinate segmentation of somites. Development 121: 1533-1545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
