Notch3 is required for arterial identity and maturation of vascular smooth muscle cells
- PMID: 15545631
- PMCID: PMC528893
- DOI: 10.1101/gad.308904
Notch3 is required for arterial identity and maturation of vascular smooth muscle cells
Abstract
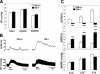
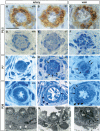
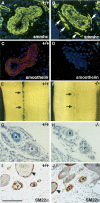
Formation of a fully functional artery proceeds through a multistep process. Here we show that Notch3 is required to generate functional arteries in mice by regulating arterial differentiation and maturation of vascular smooth muscle cells (vSMC). In adult Notch3-/- mice distal arteries exhibit structural defects and arterial myogenic responses are defective. The postnatal maturation stage of vSMC is deficient in Notch3-/- mice. We further show that Notch3 is required for arterial specification of vSMC but not of endothelial cells. Our data reveal Notch3 to be the first cell-autonomous regulator of arterial differentiation and maturation of vSMC.
Figures


References
-
- Artavanis-Tsakonas S., Rand, M.D., and Lake, R.J. 1999. Notch signaling: Cell fate control and signal integration in development. Science 284: 770-776. - PubMed
-
- Berrou E. and Bryckaert, M. 2001. Platelet-derived growth factor inhibits smooth muscle cell adhesion to fibronectin by ERK-dependent and ERK-independent pathways. J. Biol. Chem. 276: 39303-39309. - PubMed
-
- Bonvento G., Charbonne, R., Correze, J.L., Borredon, J., Seylaz, J., and Lacombe, P. 1994. Is α-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res. 665: 213-221. - PubMed
-
- Carmeliet P. 2003. Angiogenesis in health and disease. Nat. Med. 9: 653-660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
