c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation
- PMID: 15545632
- PMCID: PMC528895
- DOI: 10.1101/gad.313104
c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation
Abstract
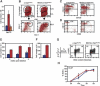
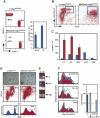
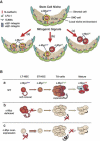
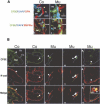
The activity of adult stem cells is essential to replenish mature cells constantly lost due to normal tissue turnover. By a poorly understood mechanism, stem cells are maintained through self-renewal while concomitantly producing differentiated progeny. Here, we provide genetic evidence for an unexpected function of the c-Myc protein in the homeostasis of hematopoietic stem cells (HSCs). Conditional elimination of c-Myc activity in the bone marrow (BM) results in severe cytopenia and accumulation of HSCs in situ. Mutant HSCs self-renew and accumulate due to their failure to initiate normal stem cell differentiation. Impaired differentiation of c-Myc-deficient HSCs is linked to their localization in the differentiation preventative BM niche environment, and correlates with up-regulation of N-cadherin and a number of adhesion receptors, suggesting that release of HSCs from the stem cell niche requires c-Myc activity. Accordingly, enforced c-Myc expression in HSCs represses N-cadherin and integrins leading to loss of self-renewal activity at the expense of differentiation. Endogenous c-Myc is differentially expressed and induced upon differentiation of long-term HSCs. Collectively, our data indicate that c-Myc controls the balance between stem cell self-renewal and differentiation, presumably by regulating the interaction between HSCs and their niche.
Figures


References
-
- Adolfsson J., Borge, O.J., Bryder, D., Theilgaard-Monch, K., Astrand-Grundstrom, I., Sitnicka, E., Sasaki, Y., and Jacobsen, S.E. 2001. Upregulation of Flt3 expression within the bone marrow Lin(–)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15: 659-669. - PubMed
-
- Akashi K., Kondo, M., Cheshier, S., Shizuru, J., Gandy, K., Domen, J., Mebius, R., Traver, D., and Weissman, I.L. 1999. Lymphoid development from stem cells and the common lymphocyte progenitors. Cold Spring Harb. Symp. Quant. Biol. 64: 1-12. - PubMed
-
- Akashi K., Traver, D., Miyamoto, T., and Weissman, I.L. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193-197. - PubMed
-
- Amati B., Alevizopoulos, K., and Vlach, J. 1998. Myc and the cell cycle. Front. Biosci. 3: D250-D268. - PubMed
-
- Arai F., Hirao, A., Ohmura, M., Sato, H., Matsuoka, S., Takubo, K., Ito, K., Koh, G.Y., and Suda, T. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149-161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
