A chaperone network controls the heat shock response in E. coli
- PMID: 15545634
- PMCID: PMC528900
- DOI: 10.1101/gad.1219204
A chaperone network controls the heat shock response in E. coli
Abstract
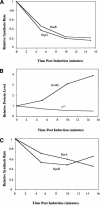
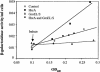
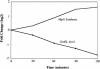
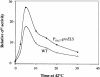
The heat shock response controls levels of chaperones and proteases to ensure a proper cellular environment for protein folding. In Escherichia coli, this response is mediated by the bacterial-specific transcription factor, sigma32. The DnaK chaperone machine regulates both the amount and activity of sigma32, thereby coupling sigma32 function to the cellular protein folding state. In this manuscript, we analyze the ability of other major chaperones in E. coli to regulate sigma32, and we demonstrate that the GroEL/S chaperonin is an additional regulator of sigma32. We show that increasing the level of GroEL/S leads to a decrease in sigma32 activity in vivo and this effect can be eliminated by co-overexpression of a GroEL/S-specific substrate. We also show that depletion of GroEL/S in vivo leads to up-regulation of sigma32 by increasing the level of sigma32. In addition, we show that changing the levels of GroEL/S during stress conditions leads to measurable changes in the heat shock response. Using purified proteins, we show that that GroEL binds to sigma32 and decreases sigma32-dependent transcription in vitro, suggesting that this regulation is direct. We discuss why using a chaperone network to regulate sigma32 results in a more sensitive and accurate detection of the protein folding environment.
Figures


Similar articles
-
The heat shock response of Escherichia coli.Int J Food Microbiol. 2000 Apr 10;55(1-3):3-9. doi: 10.1016/s0168-1605(00)00206-3. Int J Food Microbiol. 2000. PMID: 10791710 Review.
-
Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli.Mol Microbiol. 1998 Nov;30(3):567-81. doi: 10.1046/j.1365-2958.1998.01090.x. Mol Microbiol. 1998. PMID: 9822822
-
ClpB and HtpG facilitate de novo protein folding in stressed Escherichia coli cells.Mol Microbiol. 2000 Jun;36(6):1360-70. doi: 10.1046/j.1365-2958.2000.01951.x. Mol Microbiol. 2000. PMID: 10931286
-
A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor sigma32.EMBO J. 1996 Feb 1;15(3):607-17. EMBO J. 1996. PMID: 8599944 Free PMC article.
-
Substrate Interaction Networks of the Escherichia coli Chaperones: Trigger Factor, DnaK and GroEL.Adv Exp Med Biol. 2015;883:271-94. doi: 10.1007/978-3-319-23603-2_15. Adv Exp Med Biol. 2015. PMID: 26621473 Review.
Cited by
-
Regulation of bacterial heat shock stimulons.Cell Stress Chaperones. 2016 Nov;21(6):959-968. doi: 10.1007/s12192-016-0727-z. Epub 2016 Aug 12. Cell Stress Chaperones. 2016. PMID: 27518094 Free PMC article. Review.
-
Global gene expression analysis of the heat shock response in the phytopathogen Xylella fastidiosa.J Bacteriol. 2006 Aug;188(16):5821-30. doi: 10.1128/JB.00182-06. J Bacteriol. 2006. PMID: 16885450 Free PMC article.
-
Expression and assembly of a functional type IV secretion system elicit extracytoplasmic and cytoplasmic stress responses in Escherichia coli.J Bacteriol. 2006 Sep;188(18):6611-21. doi: 10.1128/JB.00632-06. J Bacteriol. 2006. PMID: 16952953 Free PMC article.
-
Gene expression profiling of intrinsic thermotolerance in Escherichia coli.Curr Microbiol. 2006 Jan;52(1):50-4. doi: 10.1007/s00284-005-4578-6. Epub 2006 Jan 2. Curr Microbiol. 2006. PMID: 16392005
-
Growth phase- and cell division-dependent activation and inactivation of the {sigma}32 regulon in Escherichia coli.J Bacteriol. 2009 Mar;191(5):1695-702. doi: 10.1128/JB.01536-08. Epub 2008 Dec 29. J Bacteriol. 2009. PMID: 19114495 Free PMC article.
References
-
- Arsene F., Tomoyasu, T., and Bukau, B. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55: 3-9. - PubMed
-
- Bahl H., Echols, H., Straus, D.B., Court, D., Crowl, R., and Georgopoulos, C.P. 1987. Induction of the heat shock response of E. coli through stabilization of σ32 by the phage lambda cIII protein. Genes & Dev. 1: 57-64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
