Meiotic recombination in Drosophila females depends on chromosome continuity between genetically defined boundaries
- PMID: 15545646
- PMCID: PMC1449117
- DOI: 10.1534/genetics.104.035824
Meiotic recombination in Drosophila females depends on chromosome continuity between genetically defined boundaries
Abstract
In the pairing-site model, specialized regions on each chromosome function to establish meiotic homolog pairing. Analysis of these sites could provide insights into the mechanism used by Drosophila females to form a synaptonemal complex (SC) in the absence of meiotic recombination. These specialized sites were first established on the X chromosome by noting that there were barriers to crossover suppression caused by translocation heterozygotes. These sites were genetically mapped and proposed to be pairing sites. By comparing the cytological breakpoints of third chromosome translocations to their patterns of crossover suppression, we have mapped two sites on chromosome 3R. We have performed experiments to determine if these sites have a role in meiotic homolog pairing and the initiation of recombination. Translocation heterozygotes exhibit reduced gene conversion within the crossover-suppressed region, consistent with an effect on the initiation of meiotic recombination. To determine if homolog pairing is disrupted in translocation heterozygotes, we used fluorescent in situ hybridization to measure the extent of homolog pairing. In wild-type oocytes, homologs are paired along their entire lengths prior to accumulation of the SC protein C(3)G. Surprisingly, translocation heterozygotes exhibited homolog pairing similar to wild type within the crossover-suppressed regions. This result contrasted with our observations of c(3)G mutant females, which were found to be defective in pairing. We propose that each Drosophila chromosome is divided into several domains by specialized sites. These sites are not required for homolog pairing. Instead, the initiation of meiotic recombination requires continuity of the meiotic chromosome structure within each of these domains.
Figures


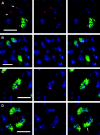

References
-
- Baker, B. S., A. T. C. Carpenter, M. S. Esposito, R. E. Esposito and L. Sandler, 1976. The genetic control of meiosis. Annu. Rev. Genet. 10: 53–134. - PubMed
-
- Baudat, F., K. Manova, J. P. Yuen, M. Jasin and S. Keeney, 2000. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6: 989–998. - PubMed
-
- Bhagat, R., E. A. Manheim, D. E. Sherizen and K. S. McKim, 2004. Studies on crossover specific mutants and the distribution of crossing over in Drosophila females. Cytogenet. Genome Res. 107: 160–171. - PubMed
-
- Carpenter, A. T. C., 1975. Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma 51: 157–182. - PubMed
-
- Carpenter, A. T. C., 1988 Thoughts on recombination nodules, meiotic recombination, and chiasmata, pp. 529–548 in Genetic Recombination, edited by R. Kucherlapati and G. Smith. American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

