Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells
- PMID: 15545975
- PMCID: PMC2409767
- DOI: 10.1038/sj.bjc.6602215
Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells
Abstract
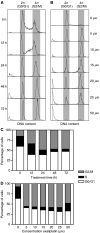
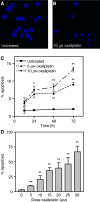
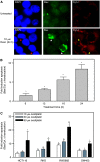
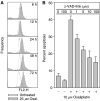
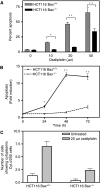
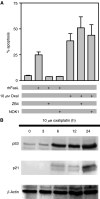
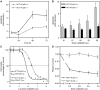
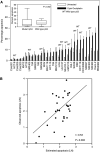
The platinum compound oxaliplatin has been shown to be an effective chemotherapeutic agent for the treatment of colorectal cancer. In this study, we investigate the molecular mechanisms of action of oxaliplatin to identify means of predicting response to this agent. Exposure of colon cancer cells to oxaliplatin resulted in G2/M arrest and apoptosis. Immunofluorescent staining demonstrated that the apoptotic cascade initiated by oxaliplatin is characterised by translocation of Bax to the mitochondria and cytochrome c release into the cytosol. Oxaliplatin treatment resulted in caspase 3 activation and oxaliplatin-induced apoptosis was abrogated by inhibition of caspase activity with z-VAD-fmk, but was independent of Fas/FasL association. Targeted inactivation of Bax or p53 in HCT116 cells resulted in significantly increased resistance to oxaliplatin. However, the mutational status of p53 was unable to predict response to oxaliplatin in a panel of 30 different colorectal cancer cell lines. In contrast, the expression profile of these 30 cell lines, assessed using a 9216-sequence cDNA microarray, successfully predicted the apoptotic response to oxaliplatin. A leave-one-out cross-validation approach was used to demonstrate a significant correlation between experimentally observed and expression profile predicted apoptosis in response to clinically achievable doses of oxaliplatin (R=0.53; P=0.002). In addition, these microarray experiments identified several genes involved in control of apoptosis and DNA damage repair that were significantly correlated with response to oxaliplatin.
Figures
References
-
- Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17: 3560–3568 - PubMed
-
- Arango D, Augenlicht LH (2001) New approaches to colorectal cancer treatment. In Recent Research Developments in Cancer Pandalal SG (ed). Vol 3, pp 385–395. Trivandrum: Transworld Research Network
-
- Arango D, Corner GA, Wadler S, Catalano PJ, Augenlicht LH (2001) c-myc/p53 interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res 61: 4910–4915 - PubMed
-
- Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S (2003) Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer 39: 112–119 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

