Stem cells: science, policy, and ethics
- PMID: 15545983
- PMCID: PMC525749
- DOI: 10.1172/JCI23549
Stem cells: science, policy, and ethics
Abstract
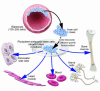
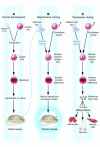
Human embryonic stem cells offer the promise of a new regenerative medicine in which damaged adult cells can be replaced with new cells. Research is needed to determine the most viable stem cell lines and reliable ways to promote the differentiation of pluripotent stem cells into specific cell types (neurons, muscle cells, etc). To create new cell lines, it is necessary to destroy preimplantation blastocysts. This has led to an intense debate that threatens to limit embryonic stem cell research. The profound ethical issues raised call for informed, dispassionate debate.
Figures

References
-
- Evers BM, Weissman IL, Flake AW, Tabar V, Weisel RD. Stem cells in clinical practice. J. Am. Coll. Surg. 2003;197:458–478. - PubMed
-
- Raff M. Adult stem cell plasticity: fact or artifact? Annu. Rev. Cell Dev. Biol. 2003;19:1–22. - PubMed
-
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. - PubMed
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
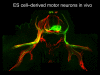
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

