Foxo1 mediates insulin action on apoC-III and triglyceride metabolism
- PMID: 15546000
- PMCID: PMC525736
- DOI: 10.1172/JCI19992
Foxo1 mediates insulin action on apoC-III and triglyceride metabolism
Abstract
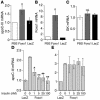
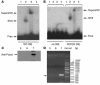
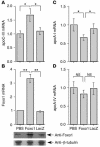
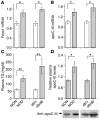
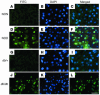
The apolipoprotein apoC-III plays an important role in plasma triglyceride metabolism. It is predominantly produced in liver, and its hepatic expression is inhibited by insulin. To elucidate the inhibitory mechanism of insulin in apoC-III expression, we delivered forkhead box O1 (Foxo1) cDNA to hepatocytes by adenovirus-mediated gene transfer. Foxo1 stimulated hepatic apoC-III expression and correlated with the ability of Foxo1 to bind to its consensus site in the apoC-III promoter. Deletion or mutation of the Foxo1 binding site abolished insulin response and Foxo1-mediated stimulation. Likewise, Foxo1 also mediated insulin action on intestinal apoC-III expression in enterocytes. Furthermore, elevated Foxo1 production in liver augmented hepatic apoC-III expression, resulting in increased plasma triglyceride levels and impaired fat tolerance in mice. Transgenic mice expressing a constitutively active Foxo1 allele exhibited hypertriglyceridemia. Moreover, we show that hepatic Foxo1 expression becomes deregulated as a result of insulin deficiency or insulin resistance, culminating in significantly elevated Foxo1 production, along with its skewed nuclear distribution, in livers of diabetic NOD or db/db mice. While loss of insulin response is associated with unrestrained apoC-III production and impaired triglyceride metabolism, these data suggest that Foxo1 provides a molecular link between insulin deficiency or resistance and aberrant apoC-III production in the pathogenesis of diabetic hypertriglyceridemia.
Figures



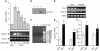
References
-
- McConathy WJ, Gesquiere JC, Bass H, Tartar A, Fruchart JC. Inhibition of lipoprotein lipase activity by synthetic peptides of apolipoprotein C-III. J. Lipid Res. 1992;33:995–1003. - PubMed
-
- Kinnunen PKJ, Ehnholm C. Effect of serum and C apolipoproteins from very low density lipoproteins on human post-heparin plasma hepatic lipase. FEBS Lett. 1976;65:354–357. - PubMed
-
- Wuarfordt SH, Michalopoulos G, Schirmer B. The effect of human C apolipoproteins on the in vitro hepatic metabolism of triglyceride emulsions in the rat. J. Biol. Chem. 1982;257:14642–14647. - PubMed
-
- Windler E, Havel RJ. Inhibitory effect of C apolipoprotein from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J. Lipid Res. 1985;26:556–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

