Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels
- PMID: 15546952
- PMCID: PMC4757428
- DOI: 10.1182/blood-2004-04-1566
Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels
Abstract
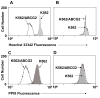
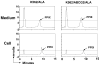
ABCG2/BCRP is a member of the adenosine triphosphate-binding cassette (ABC) transporter family and is expressed in intestine, kidney, and liver, where it modulates the absorption and excretion of xenobiotic compounds. ABCG2 is also expressed in hematopoietic stem cells and erythroid cells; however, little is known regarding its role in hematopoiesis. Abcg2 null mice have increased levels of protoporphyrin IX (PPIX) in erythroid cells, yet the mechanism for this remains uncertain. We have found that Abcg2 mRNA expression was up-regulated in differentiating erythroid cells, coinciding with increased expression of other erythroid-specific genes. This expression pattern was associated with significant amounts of ABCG2 protein on the membrane of mature peripheral blood erythrocytes. Erythroid cells engineered to express ABCG2 had significantly lower intracellular levels of PPIX, suggesting the modulation of PPIX level by ABCG2. This modulating activity was abrogated by treatment with a specific ABCG2 inhibitor, Ko143, implying that PPIX may be a direct substrate for the transporter. Taken together, our results demonstrate that ABCG2 plays a role in regulating PPIX levels during erythroid differentiation and suggest a potential role for ABCG2 as a genetic determinant in erythropoietic protoporphyria.
Figures


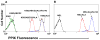
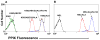

Similar articles
-
The essential role of the transporter ABCG2 in the pathophysiology of erythropoietic protoporphyria.Sci Adv. 2019 Sep 18;5(9):eaaw6127. doi: 10.1126/sciadv.aaw6127. eCollection 2019 Sep. Sci Adv. 2019. PMID: 31555729 Free PMC article.
-
Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation.PLoS One. 2012;7(11):e50082. doi: 10.1371/journal.pone.0050082. Epub 2012 Nov 26. PLoS One. 2012. PMID: 23189181 Free PMC article.
-
Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells.Mol Cell Biochem. 2011 Dec;358(1-2):297-307. doi: 10.1007/s11010-011-0980-5. Epub 2011 Jul 12. Mol Cell Biochem. 2011. PMID: 21748335
-
Roles of the ABCG2 Transporter in Protoporphyrin IX Distribution and Toxicity.Drug Metab Dispos. 2024 Oct 16;52(11):1201-1207. doi: 10.1124/dmd.123.001582. Drug Metab Dispos. 2024. PMID: 38351044 Review.
-
Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor.Adv Cancer Res. 2015;125:197-216. doi: 10.1016/bs.acr.2014.11.008. Epub 2015 Jan 8. Adv Cancer Res. 2015. PMID: 25640271 Review.
Cited by
-
ABCG2 transports and transfers heme to albumin through its large extracellular loop.J Biol Chem. 2010 Oct 22;285(43):33123-33133. doi: 10.1074/jbc.M110.139170. Epub 2010 Aug 12. J Biol Chem. 2010. PMID: 20705604 Free PMC article.
-
Coordinated missplicing of TMEM14C and ABCB7 causes ring sideroblast formation in SF3B1-mutant myelodysplastic syndrome.Blood. 2022 Mar 31;139(13):2038-2049. doi: 10.1182/blood.2021012652. Blood. 2022. PMID: 34861039 Free PMC article.
-
Mechanistic study of PpIX accumulation using the JFCR39 cell panel revealed a role for dynamin 2-mediated exocytosis.Sci Rep. 2019 Jun 17;9(1):8666. doi: 10.1038/s41598-019-44981-y. Sci Rep. 2019. PMID: 31209282 Free PMC article.
-
OATP1B1/1B3 deficiency exacerbates hyperbilirubinemia in erythropoietic protoporphyria.Drug Metab Dispos. 2025 Jul;53(7):100105. doi: 10.1016/j.dmd.2025.100105. Epub 2025 May 27. Drug Metab Dispos. 2025. PMID: 40540978 Free PMC article.
-
Medically Important Alterations in Transport Function and Trafficking of ABCG2.Int J Mol Sci. 2021 Mar 10;22(6):2786. doi: 10.3390/ijms22062786. Int J Mol Sci. 2021. PMID: 33801813 Free PMC article. Review.
References
-
- Jonker JW, Smit JW, Brinkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. - PubMed
-
- Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

