Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells
- PMID: 15547113
- PMCID: PMC4133138
- DOI: 10.1152/ajprenal.00380.2004
Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells
Abstract
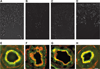
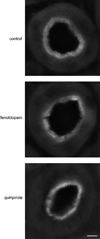
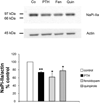
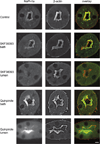
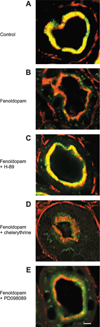
The Na(+)/phosphate cotransporter NaPi-IIa (SLC34A1) is the major transporter mediating the reabsorption of P(i) in the proximal tubule. Expression and activity of NaPi-IIa is regulated by several factors, including parathyroid hormone, dopamine, metabolic acidosis, and dietary P(i) intake. Dopamine induces natriuresis and phosphaturia in vivo, and its actions on several Na(+)-transporting systems such as NHE3 and Na(+)-K(+)-ATPase have been investigated in detail. Using freshly isolated mouse kidney slices, perfused proximal tubules, and cultured renal epithelial cells, we examined the acute effects of dopamine on NaPi-IIa expression and localization. Incubation of isolated kidney slices with the selective D(1)-like receptor agonists fenoldopam (10 microM) and SKF-38393 (10 microM) for 1 h induced NaPi-IIa internalization and reduced expression of NaPi-IIa in the brush border membrane (BBM). The D(2)-like selective agonist quinpirole (1 microM) had no effect. The D(1) and D(2) agonists did not affect the renal Na(+)/sulfate cotransporter NaSi in the BBM of the proximal tubule. Studies with isolated perfused proximal tubules demonstrated that activation of luminal, but not basolateral, D(1)-like receptors caused NaPi-IIa internalization. In kidney slices, inhibition of PKC (1 microM chelerythrine) or ERK1/2 (20 microM PD-098089) pathways did not prevent the fenoldopam-induced internalization. Inhibition with the PKA blocker H-89 (10 microM) abolished the effect of fenoldopam. Immunoblot demonstrated a reduction of NaPi-IIa protein in BBMs from kidney slices treated with fenoldopam. Incubation of opossum kidney cells transfected with NaPi-IIa-green fluorescent protein chimera shifted fluorescence from the apical membrane to an intracellular pool. In summary, dopamine induces internalization of NaPi-IIa by activation of luminal D(1)-like receptors, an effect that is mediated by PKA.
Figures

References
-
- Arar M, Baum M, Biber J, Murer H, Levi M. Epidermal growth factor inhibits Na-Pi cotransport and mRNA in OK cells. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F309–F314. - PubMed
-
- Bachmann S, Schlichting U, Geist B, Mutig K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H, Willnow TE. Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa) J Am Soc Nephrol. 2004;15:892–900. - PubMed
-
- Bacic D, Capuano P, Gisler SM, Pribanic S, Christensen EI, Biber J, Loffing J, Kaissling B, Wagner CA, Murer H. Impaired PTHinduced endocytotic down-regulation of the renal type IIa Na+/Pi-cotransporter in RAP deficient mice with reduced megalin expression. Pflügers Arch. 2003;446:475–484. - PubMed
-
- Bacic D, Hernando N, Traebert M, Lederer E, Völkl H, Biber J, Kaissling B, Murer H. Regulation of the renal type IIa Na/Pi cotransporter by cGMP. Pflügers Arch. 2001;443:306–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

