Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid
- PMID: 15547257
- PMCID: PMC529093
- DOI: 10.1128/JB.186.23.7865-7873.2004
Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid
Abstract
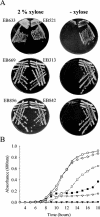
Wall teichoic acids are anionic, phosphate-rich polymers linked to the peptidoglycan of gram-positive bacteria. In Bacillus subtilis, the predominant wall teichoic acid types are poly(glycerol phosphate) in strain 168 and poly(ribitol phosphate) in strain W23, and they are synthesized by the tag and tar gene products, respectively. Growing evidence suggests that wall teichoic acids are essential in B. subtilis; however, it is widely believed that teichoic acids are dispensable under phosphate-limiting conditions. In the work reported here, we carefully studied the dispensability of teichoic acid under phosphate-limiting conditions by constructing three new mutants. These strains, having precise deletions in tagB, tagF, and tarD, were dependent on xylose-inducible complementation from a distal locus (amyE) for growth. The tarD deletion interrupted poly(ribitol phosphate) synthesis in B. subtilis and represents a unique deletion of a tar gene. When teichoic acid biosynthetic proteins were depleted, the mutants showed a coccoid morphology and cell wall thickening. The new wall teichoic acid biogenesis mutants generated in this work and a previously reported tagD mutant were not viable under phosphate-limiting conditions in the absence of complementation. Cell wall analysis of B. subtilis grown under phosphate-limited conditions showed that teichoic acid contributed approximately one-third of the wall anionic content. These data suggest that wall teichoic acid has an essential function in B. subtilis that cannot be replaced by teichuronic acid.
Figures



References
-
- Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8:115-118.
-
- Araki, Y., and E. Ito. 1989. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 17:121-135. - PubMed
-
- Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 183:6688-6693. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

