Kinetic analysis of the oxidative conversion of the [4Fe-4S]2+ cluster of FNR to a [2Fe-2S]2+ Cluster
- PMID: 15547274
- PMCID: PMC529072
- DOI: 10.1128/JB.186.23.8018-8025.2004
Kinetic analysis of the oxidative conversion of the [4Fe-4S]2+ cluster of FNR to a [2Fe-2S]2+ Cluster
Abstract
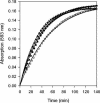
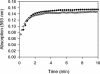
The ability of FNR to sense and respond to cellular O(2) levels depends on its [4Fe-4S](2+) cluster. In the presence of O(2), the [4Fe-4S](2+) cluster is converted to a [2Fe-2S](2+) cluster, which inactivates FNR as a transcriptional regulator. In this study, we demonstrate that approximately 2 Fe(2+) ions are released from the reaction of O(2) with the [4Fe-4S](2+) cluster. Fe(2+) release was then used as an assay of reaction progress to investigate the rate of [4Fe-4S](2+) to [2Fe-2S](2+) cluster conversion in vitro. We also found that there was no detectable difference in the rate of O(2)-induced cluster conversion for FNR free in solution compared to its DNA-bound form. In addition, the rate of FNR inactivation was monitored in vivo by measuring the rate at which transcriptional regulation by FNR is lost upon the exposure of cells to O(2); a comparison of the in vitro and in vivo rates of conversion suggests that O(2)-induced cluster conversion is sufficient to explain FNR inactivation in cells. FNR protein levels were also compared for cells grown under aerobic and anaerobic conditions.
Figures





References
-
- Agashe, V. R., S. Guha, H. C. Chang, P. Genevaux, M. Hayer-Hartl, M. Stemp, C. Georgopoulos, F. U. Hartl, and J. M. Barral. 2004. Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117:199-209. - PubMed
-
- Bates, D. M. 1999. Role of iron-sulfur cluster conversion in the oxygen-sensing mechanism of the Escherichia coli transcription factor FNR. Ph.D. thesis. University of Wisconsin—Madison, Madison.
-
- Bates, D. M., C. V. Popescu, N. Khoroshilova, K. Vogt, H. Beinert, E. Münck, and P. J. Kiley. 2000. Substitution of leucine 28 with histidine in the Escherichia coli transcription factor FNR results in increased stability of the [4Fe-4S]2+ cluster to oxygen. J. Biol. Chem. 275:6234-6240. - PubMed
-
- Beinert, H. 1983. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal. Biochem. 131:373-378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

