Effector-mediated interaction of CbbRI and CbbRII regulators with target sequences in Rhodobacter capsulatus
- PMID: 15547275
- PMCID: PMC529060
- DOI: 10.1128/JB.186.23.8026-8035.2004
Effector-mediated interaction of CbbRI and CbbRII regulators with target sequences in Rhodobacter capsulatus
Abstract
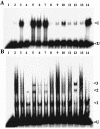
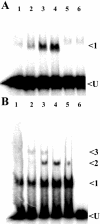
In Rhodobacter capsulatus, genes encoding enzymes of the Calvin-Benson-Bassham reductive pentose phosphate pathway are located in the cbb(I) and cbb(II) operons. Each operon contains a divergently transcribed LysR-type transcriptional activator (CbbR(I) and CbbR(II)) that regulates the expression of its cognate cbb promoter in response to an as yet unidentified effector molecule(s). Both CbbR(I) and CbbR(II) were purified, and the ability of a variety of potential effector molecules to induce changes in their DNA binding properties at their target promoters was assessed. The responses of CbbR(I) and CbbR(II) to potential effectors were not identical. In gel mobility shift assays, the affinity of both CbbR(I) and CbbR(II) for their target promoters was enhanced in the presence of ribulose-1,5-bisphosphate (RuBP), phosphoenolpyruvate, 3-phosphoglycerate, 2-phosphoglycolate. ATP, 2-phosphoglycerate, and KH(2)PO(4) were found to enhance only CbbR(I) binding, while fructose-1,6-bisphosphate enhanced the binding of only CbbR(II). The DNase I footprint of CbbR(I) was reduced in the presence of RuBP, while reductions in the CbbR(II) DNase I footprint were induced by fructose-1,6-bisphosphate, 3-phosphoglycerate, and KH(2)PO(4). The current in vitro results plus recent in vivo studies suggest that CbbR-mediated regulation of cbb transcription is controlled by multiple metabolic signals in R. capsulatus. This control reflects not only intracellular levels of Calvin-Benson-Bassham cycle metabolic intermediates but also the fixed (organic) carbon status and energy charge of the cell.
Figures





Similar articles
-
Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus.J Mol Biol. 2000 Jul 28;300(5):1079-99. doi: 10.1006/jmbi.2000.3914. J Mol Biol. 2000. PMID: 10903856
-
Physiological control and regulation of the Rhodobacter capsulatus cbb operons.J Bacteriol. 1998 Aug;180(16):4258-69. doi: 10.1128/JB.180.16.4258-4269.1998. J Bacteriol. 1998. PMID: 9696777 Free PMC article.
-
Metabolic signals that lead to control of CBB gene expression in Rhodobacter capsulatus.J Bacteriol. 2002 Apr;184(7):1905-15. doi: 10.1128/JB.184.7.1905-1915.2002. J Bacteriol. 2002. PMID: 11889097 Free PMC article.
-
Genetics and control of CO(2) assimilation in the chemoautotroph Ralstonia eutropha.Arch Microbiol. 2002 Aug;178(2):85-93. doi: 10.1007/s00203-002-0441-3. Epub 2002 Jun 14. Arch Microbiol. 2002. PMID: 12115053 Review.
-
CbbR, the Master Regulator for Microbial Carbon Dioxide Fixation.J Bacteriol. 2015 Nov;197(22):3488-98. doi: 10.1128/JB.00442-15. Epub 2015 Aug 31. J Bacteriol. 2015. PMID: 26324454 Free PMC article. Review.
Cited by
-
The effect of CbbR-binding affinity to the upstream of cbbF and cfxB on the metabolic effector in Rhodobacter sphaeroides.Curr Microbiol. 2015 Jun;70(6):816-20. doi: 10.1007/s00284-015-0789-7. Epub 2015 Feb 24. Curr Microbiol. 2015. PMID: 25708583
-
Regulatory system of the protocatechuate 4,5-cleavage pathway genes essential for lignin downstream catabolism.J Bacteriol. 2010 Jul;192(13):3394-405. doi: 10.1128/JB.00215-10. Epub 2010 Apr 30. J Bacteriol. 2010. PMID: 20435721 Free PMC article.
-
Unraveling RubisCO Form I and Form II Regulation in an Uncultured Organism from a Deep-Sea Hydrothermal Vent via Metagenomic and Mutagenesis Studies.Front Microbiol. 2017 Jul 12;8:1303. doi: 10.3389/fmicb.2017.01303. eCollection 2017. Front Microbiol. 2017. PMID: 28747908 Free PMC article.
-
Expression and regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase genes in Mycobacterium sp. strain JC1 DSM 3803.J Microbiol. 2009 Jun;47(3):297-307. doi: 10.1007/s12275-008-0210-3. Epub 2009 Jun 26. J Microbiol. 2009. PMID: 19557347
-
Further unraveling the regulatory twist by elucidating metabolic coinducer-mediated CbbR-cbbI promoter interactions in Rhodopseudomonas palustris CGA010.J Bacteriol. 2012 Mar;194(6):1350-60. doi: 10.1128/JB.06418-11. Epub 2012 Jan 13. J Bacteriol. 2012. PMID: 22247506 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology, vol. 1. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
-
- Bird, T. H., S. Du, and C. E. Bauer. 1999. Autophosphorylation, phospho-transfer and DNA binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem. 274:16343-16348. - PubMed
-
- Bishop, R. E., and J. H. Weiner. 1993. Overproduction, solubilization, purification and DNA binding properties of AmpR from Citrobacter freundii. Eur. J. Biochem. 213:405-412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

