Vibrio cholerae strains with mutations in an atypical type I secretion system accumulate RTX toxin intracellularly
- PMID: 15547287
- PMCID: PMC529086
- DOI: 10.1128/JB.186.23.8137-8143.2004
Vibrio cholerae strains with mutations in an atypical type I secretion system accumulate RTX toxin intracellularly
Abstract
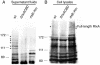
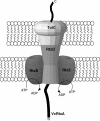
This study shows that the Vibrio cholerae RTX toxin is secreted by a four-component type I secretion system (TISS) encoded by rtxB, rtxD, rtxE, and tolC. ATP-binding site mutations in both RtxB and RtxE blocked secretion, demonstrating that this atypical TISS requires two transport ATPases that may function as a heterodimer.
Figures

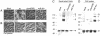

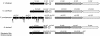
References
-
- Alberts, P., O. Daumke, E. V. Deverson, J. C. Howard, and M. R. Knittler. 2001. Distinct functional properties of the TAP subunits coordinate the nucleotide-dependent transport cycle. Curr. Biol. 11:242-251. - PubMed
-
- Alm, R. A., and P. A. Manning. 1990. Characterization of the hlyB gene and its role in the production of the El Tor haemolysin of Vibrio cholerae O1. Mol. Microbiol. 4:413-425. - PubMed
-
- Bouabe, H., and M. R. Knittler. 2003. The distinct nucleotide binding states of the transporter associated with antigen processing (TAP) are regulated by the nonhomologous C-terminal tails of TAP1 and TAP2. Eur. J. Biochem. 270:4531-4546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

