Saposin C promotes survival and prevents apoptosis via PI3K/Akt-dependent pathway in prostate cancer cells
- PMID: 15548330
- PMCID: PMC535542
- DOI: 10.1186/1476-4598-3-31
Saposin C promotes survival and prevents apoptosis via PI3K/Akt-dependent pathway in prostate cancer cells
Abstract
Background: In addition to androgens, growth factors are also implicated in the development and neoplastic growth of the prostate gland. Prosaposin is a potent neurotrophic molecule. Homozygous inactivation of prosaposin in mice has led to the development of a number of abnormalities in the male reproductive system, including atrophy of the prostate gland and inactivation of mitogen-activated protein kinase (MAPK) and Akt in prostate epithelial cells. We have recently reported that prosaposin is expressed at a higher level by androgen-independent (AI) prostate cancer cells as compared to androgen-sensitive prostate cancer cells or normal prostate epithelial and stromal cells. In addition, we have demonstrated that a synthetic peptide (prosaptide TX14A), derived from the trophic sequence of the saposin C domain of prosaposin, stimulated cell proliferation, migration and invasion and activated the MAPK signaling pathway in prostate cancer cells. The biological significances of saposin C and prosaposin in prostate cancer are not known.
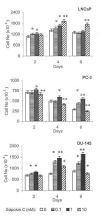
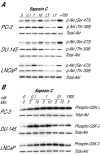
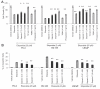
Results: Here, we report that saposin C, in a cell type-specific and dose-dependent manner, acts as a survival factor, activates the Akt-signaling pathway, down-modulates caspase-3, -7, and -9 expression and/or activity, and decreases the cleaved nuclear substrate of caspase-3 in prostate cancer cells under serum-starvation stress. In addition, prosaptide TX14A, saposin C, or prosaposin decreased the growth-inhibitory effect, caspase-3/7 activity, and apoptotic cell death induced by etoposide. We also discovered that saposin C activates the p42/44 MAP kinase pathway in a pertussis toxin-sensitive and phosphatidylinositol 3-kinase (PI3K) /Akt-dependent manner in prostate cancer cells. Our data also show that the anti-apoptotic activity of saposin C is at least partially mediated via PI3K/Akt signaling pathway.
Conclusion: We postulate that as a mitogenic, survival, and anti-apoptotic factor for prostate cancer cells, saposin C or prosaposin may contribute to prostate carcinogenesis at its early androgen-dependent or metastatic AI state.
Figures



Similar articles
-
Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells.Prostate. 2004 Feb 15;58(3):259-68. doi: 10.1002/pros.10332. Prostate. 2004. PMID: 14743465
-
Prosaptide TX14A stimulates growth, migration, and invasion and activates the Raf-MEK-ERK-RSK-Elk-1 signaling pathway in prostate cancer cells.Prostate. 2004 Oct 1;61(2):114-23. doi: 10.1002/pros.20082. Prostate. 2004. PMID: 15305334
-
Saposin C stimulates growth and invasion, activates p42/44 and SAPK/JNK signaling pathways of MAPK and upregulates uPA/uPAR expression in prostate cancer and stromal cells.Asian J Androl. 2005 Jun;7(2):147-58. doi: 10.1111/j.1745-7262.2005.00037.x. Asian J Androl. 2005. PMID: 15897971
-
Akt in prostate cancer: possible role in androgen-independence.Curr Drug Metab. 2003 Dec;4(6):487-96. doi: 10.2174/1389200033489226. Curr Drug Metab. 2003. PMID: 14683476 Review.
-
Phosphatidylinositide 3-kinase/AKT in radiation responses.Histol Histopathol. 2004 Jul;19(3):915-23. doi: 10.14670/HH-19.915. Histol Histopathol. 2004. PMID: 15168354 Review.
Cited by
-
Prosaposin: A Multifaceted Protein Orchestrating Biological Processes and Diseases.Cells. 2025 Jul 22;14(15):1131. doi: 10.3390/cells14151131. Cells. 2025. PMID: 40801564 Free PMC article. Review.
-
Prosaposin down-modulation decreases metastatic prostate cancer cell adhesion, migration, and invasion.Mol Cancer. 2010 Feb 4;9:30. doi: 10.1186/1476-4598-9-30. Mol Cancer. 2010. PMID: 20132547 Free PMC article.
-
GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin.Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9529-34. doi: 10.1073/pnas.1219004110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690594 Free PMC article.
-
Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart.Physiol Genomics. 2012 Oct 17;44(20):957-69. doi: 10.1152/physiolgenomics.00184.2011. Epub 2012 Aug 28. Physiol Genomics. 2012. PMID: 22930739 Free PMC article.
-
Role of p38 MAP Kinase Signal Transduction in Solid Tumors.Genes Cancer. 2013 Sep;4(9-10):342-59. doi: 10.1177/1947601913507951. Genes Cancer. 2013. PMID: 24349632 Free PMC article. Review.
References
-
- Kishimoto Y, Hiraiwa M, O'Brien JS. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res. 1992;33:1255–1267. - PubMed
-
- Campana WM, O'Brien JS, Hiraiwa M, Patton S. Secretion of prosaposin, a multifunctional protein, by breast cancer cells. Biochim Biophys Acta. 1999;1427:392–400. - PubMed
-
- Morimoto S, Yamamoto Y, O'Brien JS, Kishimoto Y. Determination of saposin proteins (sphingolipid activator proteins) in human tissues. Anal Biochem. 1990;190:154–157. - PubMed
-
- Chang MH, Bindloss CA, Grabowski GA, Qi X, Winchester B, Hopwood JJ, Meikle PJ. Saposins A, B, C, and D in plasma of patients with lysosomal storage disorders. Clin Chem. 2000;46:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

