Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring
- PMID: 15548368
- PMCID: PMC1531663
- DOI: 10.1593/neo.04217
Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring
Abstract
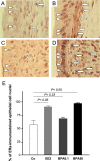
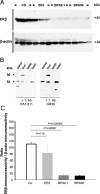
Exposure to estrogenic compounds during critical periods of fetal development could result in adverse effects on the development of reproductive organs that are not apparent until later in life. Bisphenol A (BPA), which is employed in the manufacture of a wide range of consumer products, is a prime candidate for endocrine disruption. We examined BPA to address the question of whether in utero exposure affects the uterus of the offspring and studied the expression and distribution of the estrogen receptors alpha (ERalpha) and beta (ERbeta), because estrogens influence the development, growth, and function of the uterus through both receptors. Gravid Sprague-Dawley dams were administered by gavage either 0.1 or 50 mg/kg per day BPA or 0.2 mg/kg per day 17alpha-ethinyl estradiol (EE2) as reference dose on gestation days 6 through 21. Female offspring were killed in estrus. Uterine morphologic changes as well as ERalpha and ERbeta distribution and expression were measured by immunohistochemistry and Western blot analysis. Striking morphologic changes were observed in the uterine epithelium of postpubertal offspring during estrus of the in utero BPA-treated animals (the thickness of the total epithelium was significantly reduced). ERalpha expression was increased in the 50-mg BPA and EE2-treated group. In contrast, we observed significantly decreased ERbeta expression in all BPA- and EE2-treated animals when compared with the control. In summary, these results clearly indicate that in utero exposure of rats to BPA promotes uterine disruption in offspring. We hypothesize that the uterine disruption could possibly be provoked by a dysregulation of ERalpha and ERbeta.
Figures




Similar articles
-
In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina.Neoplasia. 2002 Mar-Apr;4(2):98-102. doi: 10.1038/sj.neo.7900212. Neoplasia. 2002. PMID: 11896564 Free PMC article.
-
Estrogen receptor α and β expressions in hypothalamus-pituitary-ovary axis in rats exposed lactationally to soy isoflavones and bisphenol A.Biomed Environ Sci. 2010 Oct;23(5):357-62. doi: 10.1016/S0895-3988(10)60076-1. Biomed Environ Sci. 2010. PMID: 21112483
-
Administration of bisphenol A to dams during perinatal period modifies molecular and morphological reproductive parameters of the offspring.Reprod Toxicol. 2011 Feb;31(2):177-83. doi: 10.1016/j.reprotox.2010.10.013. Epub 2010 Nov 3. Reprod Toxicol. 2011. PMID: 21055461
-
Early programing of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies.Reprod Toxicol. 2015 Nov;57:59-72. doi: 10.1016/j.reprotox.2015.05.008. Epub 2015 May 28. Reprod Toxicol. 2015. PMID: 26028543 Free PMC article. Review.
-
Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure.Endocrinology. 2006 Jun;147(6 Suppl):S56-69. doi: 10.1210/en.2005-1159. Epub 2006 May 11. Endocrinology. 2006. PMID: 16690810 Review.
Cited by
-
Serum bisphenol S levels are associated with decreased ovarian reserve function: a single-center study.Am J Transl Res. 2024 Oct 15;16(10):5961-5969. doi: 10.62347/AQMO8416. eCollection 2024. Am J Transl Res. 2024. PMID: 39544747 Free PMC article.
-
Ancestral BPA exposure caused defects in the liver of medaka for four generations.Sci Total Environ. 2023 Jan 15;856(Pt 1):159067. doi: 10.1016/j.scitotenv.2022.159067. Epub 2022 Sep 26. Sci Total Environ. 2023. PMID: 36174697 Free PMC article.
-
Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine.Hum Reprod Update. 2022 May 2;28(3):346-375. doi: 10.1093/humupd/dmac005. Hum Reprod Update. 2022. PMID: 35187579 Free PMC article. Review.
-
Protective Effect of Aloe vera Extract against Bisphenol A Induced Testicular Toxicity in Wistar Rats.Cell J. 2018 Jul;20(2):278-283. doi: 10.22074/cellj.2018.5256. Epub 2018 Mar 18. Cell J. 2018. PMID: 29633606 Free PMC article.
-
Protective Role of Kelulut Honey against Toxicity Effects of Polystyrene Microplastics on Morphology, Hormones, and Sex Steroid Receptor Expression in the Uterus of Rats.Toxics. 2023 Mar 29;11(4):324. doi: 10.3390/toxics11040324. Toxics. 2023. PMID: 37112551 Free PMC article.
References
-
- Loder N. Royal society warns on hormone disrupters. Nature. 2000;406:4. - PubMed
-
- Triendl R. Genes may solve hormone-disrupter debate. Nature. 2001;409:274. - PubMed
-
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. - PubMed
-
- Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72:1–7. - PubMed
-
- Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143:205–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
