The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans
- PMID: 15548594
- PMCID: PMC545922
- DOI: 10.1091/mbc.e04-09-0798
The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans
Abstract
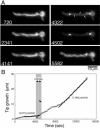
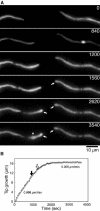
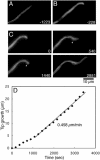
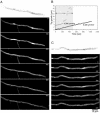
The filamentous fungus Aspergillus nidulans grows by polarized extension of hyphal tips. The actin cytoskeleton is essential for polarized growth, but the role of microtubules has been controversial. To define the role of microtubules in tip growth, we used time-lapse microscopy to measure tip growth rates in germlings of A. nidulans and in multinucleate hyphal tip cells, and we used a green fluorescent protein-alpha-tubulin fusion to observe the effects of the antimicrotubule agent benomyl. Hyphal tip cells grew approximately 5 times faster than binucleate germlings. In germlings, cytoplasmic microtubules disassembled completely in mitosis. In hyphal tip cells, however, microtubules disassembled through most of the cytoplasm in mitosis but persisted in a region near the hyphal tip. The growth rate of hyphal tip cells did not change significantly in mitosis. Benomyl caused rapid disassembly of microtubules in tip cells and a 10x reduction in growth rate. When benomyl was washed out, microtubules assembled quickly and rapid tip growth resumed. These results demonstrate that although microtubules are not strictly required for polarized growth, they are rate-limiting for the growth of hyphal tip cells. These data also reveal that A. nidulans exhibits a remarkable spatial regulation of microtubule disassembly within hyphal tip cells.
Figures

Similar articles
-
The dynamic behaviour of microtubules and their contributions to hyphal tip growth in Aspergillus nidulans.Microbiology (Reading). 2005 May;151(Pt 5):1543-1555. doi: 10.1099/mic.0.27750-0. Microbiology (Reading). 2005. PMID: 15870464
-
Aspergillus myosin-V supports polarized growth in the absence of microtubule-based transport.PLoS One. 2011;6(12):e28575. doi: 10.1371/journal.pone.0028575. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194856 Free PMC article.
-
Rapid tip-directed movement of Golgi equivalents in growing Aspergillus nidulans hyphae suggests a mechanism for delivery of growth-related materials.Microbiology (Reading). 2008 May;154(Pt 5):1544-1553. doi: 10.1099/mic.0.2007/014811-0. Microbiology (Reading). 2008. PMID: 18451063
-
Interdependence of the actin and the microtubule cytoskeleton during fungal growth.Curr Opin Microbiol. 2014 Aug;20:34-41. doi: 10.1016/j.mib.2014.04.005. Epub 2014 May 27. Curr Opin Microbiol. 2014. PMID: 24879477 Review.
-
Coordinated process of polarized growth in filamentous fungi.Biosci Biotechnol Biochem. 2016 Sep;80(9):1693-9. doi: 10.1080/09168451.2016.1179092. Epub 2016 Apr 28. Biosci Biotechnol Biochem. 2016. PMID: 27121747 Review.
Cited by
-
Off the wall: The rhyme and reason of Neurospora crassa hyphal morphogenesis.Cell Surf. 2019 Mar 8;5:100020. doi: 10.1016/j.tcsw.2019.100020. eCollection 2019 Dec. Cell Surf. 2019. PMID: 32743136 Free PMC article. Review.
-
Gamma-tubulin regulates the anaphase-promoting complex/cyclosome during interphase.J Cell Biol. 2010 Aug 9;190(3):317-30. doi: 10.1083/jcb.201002105. Epub 2010 Aug 2. J Cell Biol. 2010. PMID: 20679430 Free PMC article.
-
Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells.Sci Adv. 2018 Jan 24;4(1):e1701798. doi: 10.1126/sciadv.1701798. eCollection 2018 Jan. Sci Adv. 2018. PMID: 29387789 Free PMC article.
-
Superresolution microscopy reveals a dynamic picture of cell polarity maintenance during directional growth.Sci Adv. 2015 Nov 13;1(10):e1500947. doi: 10.1126/sciadv.1500947. eCollection 2015 Nov. Sci Adv. 2015. PMID: 26665168 Free PMC article.
-
Searching for gold beyond mitosis: Mining intracellular membrane traffic in Aspergillus nidulans.Cell Logist. 2012 Jan 1;2(1):2-14. doi: 10.4161/cl.19304. Cell Logist. 2012. PMID: 22645705 Free PMC article.
References
-
- Akashi, T., Kanbe, T., and Tanaka, K. (1994). The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology 140, 271-280. - PubMed
-
- Ålström, H., Sorri, O., and Raudaskoski, M. (1995). Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex. Plant Reprod. 8, 61-69.
-
- Anderhag, P., Hepler, P. K., and Lazzaro, M. D. (2000). Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway spruce). Protoplasma 214, 141-157.
-
- Bibikova, T. N., Blancaflor, E. B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657-665. - PubMed
-
- Clutterbuck, A. J. (1969). Cell volume per nucleus in haploid and diploid strains of Aspergillus nidulans. J. Gen. Microbiol. 55, 291-299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

