Harnessing phytochrome's glowing potential
- PMID: 15548612
- PMCID: PMC536027
- DOI: 10.1073/pnas.0407645101
Harnessing phytochrome's glowing potential
Abstract
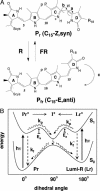
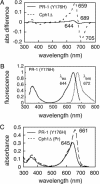
Directed evolution of a cyanobacterial phytochrome was undertaken to elucidate the structural basis of its light sensory activity by remodeling the chemical environment of its linear tetrapyrrole prosthetic group. In addition to identifying a small region of the apoprotein critical for maintaining phytochrome's native spectroscopic properties, our studies revealed a tyrosine-to-histidine mutation that transformed phytochrome into an intensely red fluorescent biliprotein. This tyrosine is conserved in all members of the phytochrome superfamily, implicating direct participation in the primary photoprocess of phytochromes. Fluorescent phytochrome mutants also hold great promise to expand the present repertoire of genetically encoded fluorescent proteins into the near infrared.
Figures
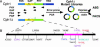


References
-
- Falk, H. (1989) The Chemistry of Linear Oligopyrroles and Bile Pigments (Springer, Vienna).
-
- Glazer, A. N. (1988) Methods Enzymol. 167, 291–303. - PubMed
-
- Smith, H. (2000) Nature 407, 585–591. - PubMed
-
- Montgomery, B. L. & Lagarias, J. C. (2002) Trends Plant Sci. 7, 357–366. - PubMed
-
- Vierstra, R. D. & Davis, S. J. (2000) Semin. Cell Dev. Biol. 11, 511–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

