Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses
- PMID: 15548614
- PMCID: PMC536018
- DOI: 10.1073/pnas.0407533101
Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses
Abstract
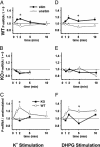
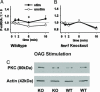
Fragile X mental retardation is caused by absence of the RNA-binding protein fragile X mental retardation protein (FMRP), encoded by the FMR1 gene. There is increasing evidence that FMRP regulates transport and modulates translation of some mRNAs. We studied neurotransmitter-activated synaptic protein synthesis in fmr1-knockout mice. Synaptoneurosomes from knockout mice did not manifest accelerated polyribosome assembly or protein synthesis as it occurs in wild-type mice upon stimulation of group I metabotropic glutamate receptors. Direct activation of protein kinase C did not compensate in the knockout mouse, indicating that the FMRP-dependent step is further along the signaling pathway. Visual cortices of young knockout mice exhibited a lower proportion of dendritic spine synapses containing polyribosomes than did the cortices of wild-type mice, corroborating this finding in vivo. This deficit in rapid neurotransmitter-controlled local translation of specific proteins may contribute to morphological and functional abnormalities observed in patients with fragile X syndrome.
Figures


Comment in
-
Fragile X syndrome: (What's) lost in translation?Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17329-30. doi: 10.1073/pnas.0408034101. Epub 2004 Dec 6. Proc Natl Acad Sci U S A. 2004. PMID: 15583122 Free PMC article. No abstract available.
Similar articles
-
Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome.J Neurosci. 2007 May 16;27(20):5338-48. doi: 10.1523/JNEUROSCI.0937-07.2007. J Neurosci. 2007. PMID: 17507556 Free PMC article.
-
Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation.Proc Natl Acad Sci U S A. 1997 May 13;94(10):5395-400. doi: 10.1073/pnas.94.10.5395. Proc Natl Acad Sci U S A. 1997. PMID: 9144248 Free PMC article.
-
The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14374-8. doi: 10.1073/pnas.2336265100. Epub 2003 Nov 12. Proc Natl Acad Sci U S A. 2003. PMID: 14614133 Free PMC article.
-
Synaptic regulation of protein synthesis and the fragile X protein.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7101-6. doi: 10.1073/pnas.141145998. Proc Natl Acad Sci U S A. 2001. PMID: 11416194 Free PMC article. Review.
-
A decade of molecular studies of fragile X syndrome.Annu Rev Neurosci. 2002;25:315-38. doi: 10.1146/annurev.neuro.25.112701.142909. Epub 2002 Mar 20. Annu Rev Neurosci. 2002. PMID: 12052912 Review.
Cited by
-
FMRP mediates mGluR5-dependent translation of amyloid precursor protein.PLoS Biol. 2007 Mar;5(3):e52. doi: 10.1371/journal.pbio.0050052. PLoS Biol. 2007. PMID: 17298186 Free PMC article.
-
A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome.J Neurosci. 2005 Sep 28;25(39):8908-16. doi: 10.1523/JNEUROSCI.0932-05.2005. J Neurosci. 2005. PMID: 16192381 Free PMC article.
-
Dynamic association of the fragile X mental retardation protein as a messenger ribonucleoprotein between microtubules and polyribosomes.Mol Biol Cell. 2008 Jan;19(1):105-14. doi: 10.1091/mbc.e07-06-0583. Epub 2007 Oct 31. Mol Biol Cell. 2008. PMID: 17978095 Free PMC article.
-
Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome.J Neurosci. 2007 May 16;27(20):5338-48. doi: 10.1523/JNEUROSCI.0937-07.2007. J Neurosci. 2007. PMID: 17507556 Free PMC article.
-
Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models.Front Synaptic Neurosci. 2010 Jun 7;2:4. doi: 10.3389/fnsyn.2010.00004. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423490 Free PMC article.
References
-
- Warren, S. T. & Nelson, D. L. (1994) J. Am. Med. Assoc. 271, 536–542. - PubMed
-
- Hagerman, R. J. & Hagerman, P. J. (2002) Fragile X Syndrome: Diagnosis, Treatment, and Research (Johns Hopkins Univ. Press, Baltimore).
-
- Hinton, V. J., Brown, W. T., Wisniewski, K. & Rudelli, R. D. (1991) Am. J. Med. Genet. 41, 289–294. - PubMed
-
- Irwin, S. A., Patel, B., Idupulapati, M., Harris, J. B., Crisostomo, R. A., Larsen, B. P., Kooy, F., Willems, P. J., Cras, P., Kozlowski, P. B., et al. (2001) Am. J. Med. Genet. 98, 161–167. - PubMed
-
- Devys, D., Lutz, Y., Rouyer, N., Bellocq, J. P. & Mandel, J. L. (1993) Nat. Genet. 4, 335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

