Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms
- PMID: 15548643
- PMCID: PMC6730292
- DOI: 10.1523/JNEUROSCI.3408-04.2004
Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms
Erratum in
- J Neurosci. 2004 Dec 15;24(50):11481
Abstract
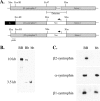
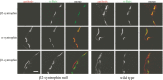
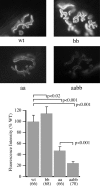
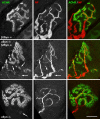
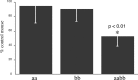
The syntrophins are modular adapter proteins that function by recruiting signaling molecules to the cytoskeleton via their direct association with proteins of the dystrophin protein family. We investigated the physiological function of beta2-syntrophin by generating a line of mice lacking this syntrophin isoform. The beta2-syntrophin null mice show no overt phenotype, or muscular dystrophy, and form structurally normal neuromuscular junctions (NMJs). To determine whether physiological consequences caused by the lack of beta2-syntrophin were masked by compensation from the alpha-syntrophin isoform, we crossed these mice with our previously described alpha-syntrophin null mice to produce mice lacking both isoforms. The alpha/beta2-syntrophin null mice have NMJs that are structurally more aberrant than those lacking only alpha-syntrophin. The NMJs of the alpha/beta2-syntrophin null mice have fewer junctional folds than either parent strain, and the remaining folds are abnormally shaped with few openings to the synaptic space. The levels of acetylcholine receptors are reduced to 23% of wild type in mice lacking both syntrophin isoforms. Furthermore, the alpha/beta2-syntrophin null mice ran significantly shorter distances on voluntary exercise wheels despite having normal neuromuscular junction transmission as determined by micro-electrode recording of endplate potentials. We conclude that both alpha-syntrophin and beta2-syntrophin play distinct roles in forming and maintaining NMJ structure and that each syntrophin can partially compensate for the loss of the other.
Figures



References
-
- Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC (1993) Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11: 531-540. - PubMed
-
- Adams ME, Dwyer TM, Dowler LL, White RA, Froehner SC (1995) Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J Biol Chem 270: 25859-25865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
