PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors
- PMID: 15548648
- PMCID: PMC6730308
- DOI: 10.1523/JNEUROSCI.3229-04.2004
PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors
Abstract
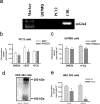
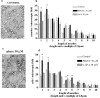
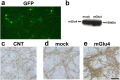
Exposure of immature rat cerebellar granule cell cultures to the type 4 metabotropic glutamate (mGlu4) receptor enhancer N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) reduced [3H]thymidine incorporation. Its action was sensitive to the growth conditions and was attenuated by two mGlu4 receptor antagonists. An antiproliferative action of PHCCC was also seen in cultures from wild-type, but not mGlu4, knock-out mice. At least in rat cultures, PHCCC was not neurotoxic and enhanced neuritogenesis. Although PHCCC reduced the increase in cAMP formation and phospho-AKT levels induced by forskolin, none of these transduction pathways significantly contributed to the reduction of [3H]thymidine incorporation. Interestingly, PHCCC reduced the expression of Gli-1, a transcription factor that mediates the mitogenic action of Sonic hedgehog. Finally, we treated newborn rats with PHCCC either intracerebrally (infusion of 5 nmol/2 microl in the cerebellar region once every other day) or systemically (5 mg/kg, i.p., once daily) from postnatal days 3-9. Local infusion of PHCCC induced substantial changes in the morphology of the developing cerebellum. In contrast, systemic injection of PHCCC induced only morphological abnormalities of the cerebellar lobule V, which became visible 11 d after the end of the treatment. These data suggest that mGlu4 receptors are involved in the regulation of cerebellar development.
Figures



Similar articles
-
Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas.J Neurosci. 2006 Aug 9;26(32):8388-97. doi: 10.1523/JNEUROSCI.2285-06.2006. J Neurosci. 2006. PMID: 16899734 Free PMC article.
-
Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors.Mol Pharmacol. 2012 May;81(5):643-56. doi: 10.1124/mol.111.074765. Epub 2012 Feb 6. Mol Pharmacol. 2012. PMID: 22311707
-
Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.J Neurosci. 2006 Jul 5;26(27):7222-9. doi: 10.1523/JNEUROSCI.1595-06.2006. J Neurosci. 2006. PMID: 16822979 Free PMC article.
-
The origin recognition complex subunit, ORC3, is developmentally regulated and supports the expression of biochemical markers of neuronal maturation in cultured cerebellar granule cells.Brain Res. 2010 Oct 28;1358:1-10. doi: 10.1016/j.brainres.2010.07.052. Epub 2010 Jul 29. Brain Res. 2010. PMID: 20674557
-
Endogenous activation of group-I metabotropic glutamate receptors is required for differentiation and survival of cerebellar Purkinje cells.J Neurosci. 2001 Oct 1;21(19):7664-73. doi: 10.1523/JNEUROSCI.21-19-07664.2001. J Neurosci. 2001. PMID: 11567056 Free PMC article.
Cited by
-
Activation of Type 4 Metabotropic Glutamate Receptor Attenuates Oxidative Stress-Induced Death of Neural Stem Cells with Inhibition of JNK and p38 MAPK Signaling.Stem Cells Dev. 2015 Nov 15;24(22):2709-22. doi: 10.1089/scd.2015.0067. Epub 2015 Sep 1. Stem Cells Dev. 2015. PMID: 26176363 Free PMC article.
-
mGluR4-positive allosteric modulation as potential treatment for Parkinson's disease.Future Med Chem. 2009 Jun;1(3):501-13. doi: 10.4155/fmc.09.38. Future Med Chem. 2009. PMID: 20161443 Free PMC article. Review.
-
Neuronal Activity in Ontogeny and Oncology.Trends Cancer. 2017 Feb;3(2):89-112. doi: 10.1016/j.trecan.2016.12.008. Epub 2017 Feb 13. Trends Cancer. 2017. PMID: 28718448 Free PMC article. Review.
-
G-protein-coupled receptors in adult neurogenesis.Pharmacol Rev. 2012 Jul;64(3):645-75. doi: 10.1124/pr.111.004762. Epub 2012 May 18. Pharmacol Rev. 2012. PMID: 22611178 Free PMC article. Review.
-
A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation.Chem Biol. 2007 Sep;14(9):1019-30. doi: 10.1016/j.chembiol.2007.07.016. Chem Biol. 2007. PMID: 17884634 Free PMC article.
References
-
- Altman J, Bayer S (1996) An overview of the postnatal development of the rat cerebellum. In: Development of the cerebellar system: in relation to its evolution, structure, and function, pp 324-333. Boca Raton, FL: CRC.
-
- Annoura H, Fukunaga A, Uesugi M, Tatuoka T, Horikawa Y (1996) A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carbozylates. Bioorg Med Chem Lett 5: 223-228.
-
- Catania MV, Landwehrmeyer GB, Testa CM, Standaert DG, Penney Jr JB, Young AB (1994) Metabotropic glutamate receptors are differentially regulated during development. Neuroscience 61: 481-495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
