Memory-like alterations in Aplysia axons after nerve injury or localized depolarization
- PMID: 15548654
- PMCID: PMC6730315
- DOI: 10.1523/JNEUROSCI.2329-04.2004
Memory-like alterations in Aplysia axons after nerve injury or localized depolarization
Abstract
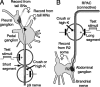
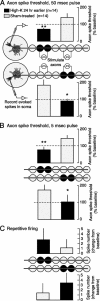
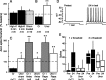
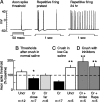
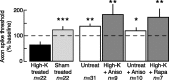
Adaptive, long-term alterations of excitability have been reported in dendrites and presynaptic terminals but not along axons. Persistent enhancement of axonal excitability has been described in proximal nerve stumps at sites of nerve section in mammals, but this hyperexcitability is considered a pathological derangement important only as a cause of neuropathic pain. Identified neurons in Aplysia were used to test the hypothesis that either axonal injury or the focal depolarization that accompanies axonal injury can trigger a local decrease in action potential threshold [long-term hyperexcitability (LTH)] having memory-like properties. Nociceptive tail sensory neurons and a giant secretomotor neuron, R2, exhibited localized axonal LTH lasting 24 hr after a crush of the nerve or connective that severed the tested axons. Axons of tail sensory neurons and tail motor neurons, but not R2, displayed similar localized LTH after peripheral depolarization produced by 2 min exposure to elevated extracellular [K(+)]. Neither the induction nor expression of either form of LTH was blocked by saline containing 1% normal [Ca(2+)] during treatment or testing. However, both were prevented by local application of the protein synthesis inhibitors anisomycin or rapamycin. The features of (1) long-lasting alteration by localized depolarization, (2) restriction of alterations to intensely depolarized regions, and (3) dependence of the alterations on local, rapamycin-sensitive protein synthesis are shared with synaptic mechanisms considered important for memory formation. This commonality suggests that relatively simple, accessible axons may offer an opportunity to define fundamental plasticity mechanisms that were important in the evolution of memory.
Figures


Similar articles
-
Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of aplysia that contributes to injury responses.J Neurophysiol. 2007 Sep;98(3):1231-9. doi: 10.1152/jn.01189.2006. Epub 2007 Jul 18. J Neurophysiol. 2007. PMID: 17634332
-
Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons.J Neurosci. 1999 Feb 15;19(4):1247-56. doi: 10.1523/JNEUROSCI.19-04-01247.1999. J Neurosci. 1999. PMID: 9952402 Free PMC article.
-
Long-lasting hyperexcitability induced by depolarization in the absence of detectable Ca2+ signals.J Neurophysiol. 2009 Mar;101(3):1351-60. doi: 10.1152/jn.91012.2008. Epub 2009 Jan 14. J Neurophysiol. 2009. PMID: 19144743 Free PMC article.
-
Long-term alterations induced by injury and by 5-HT in Aplysia sensory neurons: convergent pathways and common signals?Trends Neurosci. 1995 Mar;18(3):137-42. doi: 10.1016/0166-2236(95)93891-z. Trends Neurosci. 1995. PMID: 7754525 Review.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
Cited by
-
Induction of long-term hyperexcitability by memory-related cAMP signaling in isolated nociceptor cell bodies.bioRxiv [Preprint]. 2024 Jul 17:2024.07.13.603393. doi: 10.1101/2024.07.13.603393. bioRxiv. 2024. Update in: Neurobiol Pain. 2024 Sep 20;16:100166. doi: 10.1016/j.ynpai.2024.100166. PMID: 39071414 Free PMC article. Updated. Preprint.
-
Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
-
Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis.Front Physiol. 2012 Aug 2;3:309. doi: 10.3389/fphys.2012.00309. eCollection 2012. Front Physiol. 2012. PMID: 22934060 Free PMC article.
-
The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons.Neuroscience. 2006 Sep 15;141(4):2107-16. doi: 10.1016/j.neuroscience.2006.05.047. Epub 2006 Jun 30. Neuroscience. 2006. PMID: 16809002 Free PMC article.
-
Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR.J Neurosci. 2007 Dec 19;27(51):13958-67. doi: 10.1523/JNEUROSCI.4383-07.2007. J Neurosci. 2007. PMID: 18094233 Free PMC article.
References
-
- Baccus SA, Burrell BD, Sahley CL, Muller KJ (2000) Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol 83: 1693-1700. - PubMed
-
- Berdan RC, Easaw JC, Wang R (1993) Alterations in membrane potential after axotomy at different distances from the soma of an identified neuron and the effect of depolarization on neurite outgrowth and calcium channel expression. J Neurophysiol 69: 151-164. - PubMed
-
- Bove GM, Ransil BJ, Lin HC, Leem JG (2003) Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol 90: 1949-1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
