Multiple time scales of adaptation in auditory cortex neurons
- PMID: 15548659
- PMCID: PMC6730303
- DOI: 10.1523/JNEUROSCI.1905-04.2004
Multiple time scales of adaptation in auditory cortex neurons
Erratum in
- J Neurosci. 2005 Jan 5;25(1):2 p following 198
Abstract
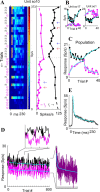
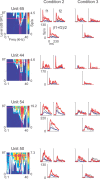
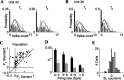
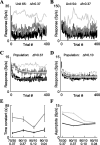
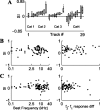
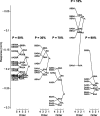
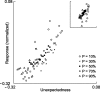
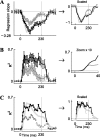
Neurons in primary auditory cortex (A1) of cats show strong stimulus-specific adaptation (SSA). In probabilistic settings, in which one stimulus is common and another is rare, responses to common sounds adapt more strongly than responses to rare sounds. This SSA could be a correlate of auditory sensory memory at the level of single A1 neurons. Here we studied adaptation in A1 neurons, using three different probabilistic designs. We showed that SSA has several time scales concurrently, spanning many orders of magnitude, from hundreds of milliseconds to tens of seconds. Similar time scales are known for the auditory memory span of humans, as measured both psychophysically and using evoked potentials. A simple model, with linear dependence on both short-term and long-term stimulus history, provided a good fit to A1 responses. Auditory thalamus neurons did not show SSA, and their responses were poorly fitted by the same model. In addition, SSA increased the proportion of failures in the responses of A1 neurons to the adapting stimulus. Finally, SSA caused a bias in the neuronal responses to unbiased stimuli, enhancing the responses to eccentric stimuli. Therefore, we propose that a major function of SSA in A1 neurons is to encode auditory sensory memory on multiple time scales. This SSA might play a role in stream segregation and in binding of auditory objects over many time scales, a property that is crucial for processing of natural auditory scenes in cats and of speech and music in humans.
Figures



References
-
- Abbott LF, Varela JA, Sen K, Nelson SB (1997) Synaptic depression and cortical gain control. Science 275: 220-224. - PubMed
-
- Bottcher-Gandor C, Ullsperger P (1992) Mismatch negativity in event-related potentials to auditory stimuli as a function of varying interstimulus interval. Psychophysiology 29: 546-550. - PubMed
-
- Bregman AS (1990) Auditory scene analysis: the perceptual organization of sound. Cambridge, MA: MIT.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
