Dendrodendritic electrical synapses between mammalian retinal ganglion cells
- PMID: 15548670
- PMCID: PMC6730298
- DOI: 10.1523/JNEUROSCI.3319-04.2004
Dendrodendritic electrical synapses between mammalian retinal ganglion cells
Abstract
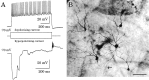
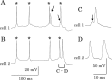
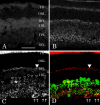
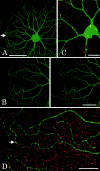
Electrical synapses between alpha-type ganglion cells were detected using combined techniques of dual patch-clamp recordings, intracellular labeling, electron microscopy, and channel subunit connexin immunocytochemistry in the albino rat retina. After intracellular injection of Neurobiotin into alpha-cells of inner (ON-center) and outer (OFF-center) ramifying types, measurement of tracer coupling resulted in a preferentially homologous occurrence among cells of the same morphological type (n = 19 of 24). In high-voltage as well as conventional electron microscopic analysis, direct dendrodendritic gap junctions (average size, 0.86 mum long) were present in contact sites between tracer-coupled alpha-cells. In simultaneous dual whole-cell recordings from pairs of neighboring alpha-cells, these cells generated TTX-sensitive sustained spiking against extrinsic current injection, and bidirectional electrical synapses (maximum coupling coefficient, 0.32) with symmetrical junction conductance (average, 1.35 nS) were observed in pairs with cells of the same morphological type. Precise temporal synchronization of spike activity (average time delay, 2.7 msec) was detected when depolarizing currents were simultaneously injected into the pairs. To address whether physiologically identified electrical synapses constitute gap junctional connectivity between cell pairs, identified neuronal connexin36 immunoreactivity was undertaken in Lucifer yellow-labeled cell pairs after patch-clamp recordings. All alpha-cells expressed connexin36, and confocal laser-scanning imaging demonstrated that connexin36 is primarily located at dendritic crossings between electrically coupled cells (seven sites in a pair, on average). These results give conclusive evidence for electrical synapses via dendrodendritic gap junctions involving connexin36 in alpha retinal ganglion cells of the same physiological type.
Figures






References
-
- Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29: 775. - PubMed
-
- Al-Ubaidi MR, White TW, Ripps H, Poras I, Avner P, Gomes D, Bruzzone R (2000) Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res 59: 813-826. - PubMed
-
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF (2000) Expression of connexin36 in the adult and developing rat brain. Brain Res 865: 121-138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
