Nucleocapsid protein as early diagnostic marker for SARS
- PMID: 15550204
- PMCID: PMC3329003
- DOI: 10.3201/eid1011.040516
Nucleocapsid protein as early diagnostic marker for SARS
Abstract
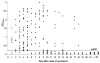
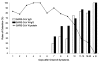
Serum samples from 317 patients with patients with severe acute respiratory syndrome (SARS) were tested for the nucleocapsid (N) protein of SARS-associated coronavirus, with sensitivities of 94% and 78% for the first 5 days and 6-10 days after onset, respectively. The specificity was 99.9%. N protein can be used as an early diagnostic maker for SARS.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
