Dissociation of slow waves and fast oscillations above 200 Hz during GABA application in rat somatosensory cortex
- PMID: 15550468
- PMCID: PMC1665326
- DOI: 10.1113/jphysiol.2004.075325
Dissociation of slow waves and fast oscillations above 200 Hz during GABA application in rat somatosensory cortex
Abstract
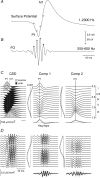
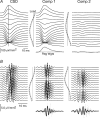
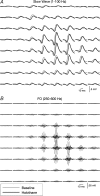
Fast electrical oscillations (FOs; > 200 Hz), superimposed on vibrissa-evoked slow potentials, may support rapid sensory integration in neocortex. Yet, while it is well established that the positive/negative (P1/N1) slow wave of the somatosensory evoked potential primarily reflects sequential activation of supragranular and infragranular pyramidal cells mediated chiefly via excitatory chemical synaptic pathways, little is known about the generation of FOs. In this study, laminar current source-density analysis and principal component analysis indicated that FOs are generated by two dipolar current sources situated in the supra- and infragranular layers, similar in laminar location to the two current dipoles associated with the slow wave. However, exogenous GABA application reversibly abolished the N1 slow wave, leaving the P1 intact, while the FO was unaffected by GABA. Furthermore, reductions in both supra- and infragranular cortical unit discharge during application of GABA suggests that FO generation is not dependent on the same intracortical synaptic circuits that are associated with the N1 slow wave. These data suggest a marked functional dissociation between neural mechanisms underlying the slow and fast components of the vibrissa-evoked response.
Figures

References
-
- Amitai Y. Thalamocortical synaptic connections: efficacy, modulation, inhibition and plasticity. Rev Neurosci. 2001;12:159–173. - PubMed
-
- Barth DS, Di S. Three-dimensional analysis of auditory-evoked potentials in rat neocortex. J Neurophysiol. 1990;64:1527–1536. - PubMed
-
- Barth DS, Di S, Baumgartner C. Laminar cortical interactions during epileptic spikes studied with principal component analysis and physiological modeling. Brain Res. 1989;484:13–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

