Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction
- PMID: 15550692
- PMCID: PMC3679649
- DOI: 10.1161/01.RES.0000150856.47324.5b
Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction
Abstract
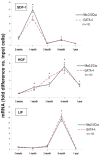
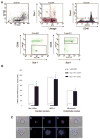
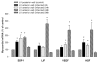
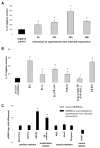
The concept that bone marrow (BM)-derived cells participate in cardiac regeneration remains highly controversial and the identity of the specific cell type(s) involved remains unknown. In this study, we report that the postnatal BM contains a mobile pool of cells that express early cardiac lineage markers (Nkx2.5/Csx, GATA-4, and MEF2C). These cells are present in significant amounts in BM harvested from young mice but their abundance decreases with age; in addition, the responsiveness of these cells to gradients of motomorphogens SDF-1, HGF, and LIF changes with age. FACS analysis, combined with analysis of early cardiac markers at the mRNA and protein levels, revealed that cells expressing these markers reside in the nonadherent, nonhematopoietic CXCR4+/Sca-1+/lin-/CD45- mononuclear cell (MNC) fraction in mice and in the CXCR4+/CD34+/AC133+/CD45- BMMNC fraction in humans. These cells are mobilized into the peripheral blood after myocardial infarction and chemoattracted to the infarcted myocardium in an SDF-1-CXCR4-, HGF-c-Met-, and LIF-LIF-R-dependent manner. To our knowledge, this is the first demonstration that the postnatal BM harbors a nonhematopoietic population of cells that express markers for cardiac differentiation. We propose that these potential cardiac progenitors may account for the myocardial regenerative effects of BM. The present findings provide a novel paradigm that could reconcile current controversies and a rationale for investigating the use of BM-derived cardiac progenitors for myocardial regeneration.
Figures

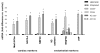
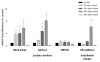
References
-
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. - PubMed
-
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. - PubMed
-
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

