Adaptive amplification and point mutation are independent mechanisms: evidence for various stress-inducible mutation mechanisms
- PMID: 15550983
- PMCID: PMC529313
- DOI: 10.1371/journal.pbio.0020399
Adaptive amplification and point mutation are independent mechanisms: evidence for various stress-inducible mutation mechanisms
Abstract
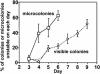
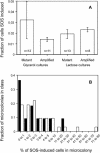
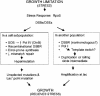
"Adaptive mutation" denotes a collection of processes in which cells respond to growth-limiting environments by producing compensatory mutants that grow well, apparently violating fundamental principles of evolution. In a well-studied model, starvation of stationary-phase lac(-)Escherichia coli cells on lactose medium induces Lac(+)revertants at higher frequencies than predicted by usual mutation models. These revertants carry either a compensatory frameshift mutation or a greater than 20-fold amplification of the leaky lac allele. A crucial distinction between alternative hypotheses for the mechanisms of adaptive mutation hinges on whether these amplification and frameshift mutation events are distinct, or whether amplification is a molecular intermediate, producing an intermediate cell type, in colonies on a pathway to frameshift mutation. The latter model allows the evolutionarily conservative idea of increased mutations (per cell) without increased mutation rate (by virtue of extra gene copies per cell), whereas the former requires an increase in mutation rate, potentially accelerating evolution. To resolve these models, we probed early events leading to rare adaptive mutations and report several results that show that amplification is not the precursor to frameshift mutation but rather is an independent adaptive outcome. (i) Using new high-resolution selection methods and stringent analysis of all cells in very young (micro)colonies (500-10,000 cells), we find that most mutant colonies contain no detectable lac-amplified cells, in contrast with previous reports. (ii) Analysis of nascent colonies, as young as the two-cell stage, revealed mutant Lac(+)cells with no lac-amplified cells present. (iii) Stringent colony-fate experiments show that microcolonies of lac-amplified cells grow to form visible colonies of lac-amplified, not mutant, cells. (iv) Mutant cells do not overgrow lac-amplified cells in microcolonies fast enough to mask the lac-amplified cells. (v)lac-amplified cells are not SOS-induced, as was proposed to explain elevated mutation in a sequential model. (vi) Amplification, and not frameshift mutation, requires DNA polymerase I, demonstrating that mutation is separable from amplification, and also illuminating the amplification mechanism. We conclude that amplification and mutation are independent outcomes of adaptive genetic change. We suggest that the availability of alternative pathways for genetic/evolutionary adaptation and clonal expansion under stress may be exploited during processes ranging from the evolution of drug resistance to cancer progression.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




References
-
- Andersson DI, Slechta ES, Roth JR. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science. 1998;282:1133–1135. - PubMed
-
- Bjedov I, Tenaillon O, Gérard B, Souza V, Denamur E, et al. Stress-induced mutagenesis in bacteria. Science. 2003;300:1404–1409. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

