Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion
- PMID: 15554962
- PMCID: PMC3869561
- DOI: 10.1111/j.1365-2958.2004.04345.x
Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion
Abstract
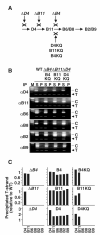
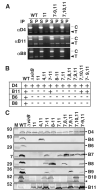
Bacteria use type IV secretion systems (T4SS) to translocate DNA (T-DNA) and protein substrates across the cell envelope. By transfer DNA immunoprecipitation (TrIP), we recently showed that T-DNA translocates through the Agrobacterium tumefaciens VirB/D4 T4SS by forming close contacts sequentially with the VirD4 receptor, VirB11 ATPase, the inner membrane subunits VirB6 and VirB8 and, finally, VirB2 pilin and VirB9. Here, by TrIP, we show that nucleoside triphosphate binding site (Walker A motif) mutations do not disrupt VirD4 substrate binding or transfer to VirB11, suggesting that these early reactions proceed independently of ATP binding or hydrolysis. In contrast, VirD4, VirB11 and VirB4 Walker A mutations each arrest substrate transfer to VirB6 and VirB8, suggesting that these subunits energize this transfer reaction by an ATP-dependent mechanism. By co-immunoprecipitation, we supply evidence for VirD4 interactions with VirB4 and VirB11 independently of other T4SS subunits or intact Walker A motifs, and with the bitopic inner membrane subunit VirB10. We reconstituted substrate transfer from VirD4 to VirB11 and to VirB6 and VirB8 by co-synthesis of previously identified 'core' components of the VirB/D4 T4SS. Our findings define genetic requirements for DNA substrate binding and the early transfer reactions of a bacterial type IV translocation pathway.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

