Prediction of quaternary assembly of SARS coronavirus peplomer
- PMID: 15555555
- PMCID: PMC7092937
- DOI: 10.1016/j.bbrc.2004.10.156
Prediction of quaternary assembly of SARS coronavirus peplomer
Abstract
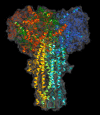
The tertiary structures of the S1 and S2 domains of the spike protein of the coronavirus which is responsible of the severe acute respiratory syndrome (SARS) have been recently predicted. Here a molecular assembly of SARS coronavirus peplomer which accounts for the available functional data is suggested. The interaction between S1 and S2 appears to be stabilised by a large hydrophobic network of aromatic side chains present in both domains. This feature results to be common to all coronaviruses, suggesting potential targeting for drugs preventing coronavirus-related infections.
Figures


References
-
- Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H., Gao G.F., Anand K., Bartlam M., Hilgenfeld R., Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA. 2003;100:13190–13195. - PMC - PubMed
-
- Egloff M.P., Ferron F., Campanacci V., Longhi S., Rancurel C., Dutartre H., Snijder E.J., Gorbalenya A.E., Cambillau C., Canard B. The severe acute respiratory syndrome-coronavirus replicative protein nsp9 is a single-stranded RNA-binding subunit unique in the RNA virus world. Proc. Natl. Acad. Sci. USA. 2004;101:3792–3796. - PMC - PubMed
-
- Shen X., Xue J.H., Yu C.Y., Luo H.B., Qin L., Yu X.J., Chen J., Chen L.L., Xiong B., Yue L.D., Cai J.H., Shen J.H., Luo X.M., Chen K.X., Shi T.L., Li Y.X., Hu G.X., Jiang H.L. Small envelope protein E of SARS: cloning, expression, purification, CD determination, and bioinformatics analysis. Acta Pharmacol. Sin. 2003;24:505–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

