Halogen bonds in biological molecules
- PMID: 15557000
- PMCID: PMC529416
- DOI: 10.1073/pnas.0407607101
Halogen bonds in biological molecules
Abstract
Short oxygen-halogen interactions have been known in organic chemistry since the 1950s and recently have been exploited in the design of supramolecular assemblies. The present survey of protein and nucleic acid structures reveals similar halogen bonds as potentially stabilizing inter- and intramolecular interactions that can affect ligand binding and molecular folding. A halogen bond in biomolecules can be defined as a short C-X...O-Y interaction (C-X is a carbon-bonded chlorine, bromine, or iodine, and O-Y is a carbonyl, hydroxyl, charged carboxylate, or phosphate group), where the X...O distance is less than or equal to the sums of the respective van der Waals radii (3.27 A for Cl...O, 3.37 A for Br...O, and 3.50 A for I...O) and can conform to the geometry seen in small molecules, with the C-X...O angle approximately 165 degrees (consistent with a strong directional polarization of the halogen) and the X...O-Y angle approximately 120 degrees . Alternative geometries can be imposed by the more complex environment found in biomolecules, depending on which of the two types of donor systems are involved in the interaction: (i) the lone pair electrons of oxygen (and, to a lesser extent, nitrogen and sulfur) atoms or (ii) the delocalized pi -electrons of peptide bonds or carboxylate or amide groups. Thus, the specific geometry and diversity of the interacting partners of halogen bonds offer new and versatile tools for the design of ligands as drugs and materials in nanotechnology.
Figures
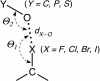
 Y can be a carbonyl, hydroxyl, or carboxylate when Y is a carbon; a phosphate when Y is a phosphorus; or a sulfate when Y is a sulfur). The geometry of the interaction is defined by the normalized RX···O distance [RX···O = dX···O/RvdW(X···O)], the Θ1 angle of the oxygen relative to the C
Y can be a carbonyl, hydroxyl, or carboxylate when Y is a carbon; a phosphate when Y is a phosphorus; or a sulfate when Y is a sulfur). The geometry of the interaction is defined by the normalized RX···O distance [RX···O = dX···O/RvdW(X···O)], the Θ1 angle of the oxygen relative to the C X bond, and the Θ2 angle of the halogen relative to the O
X bond, and the Θ2 angle of the halogen relative to the O Y bond.
Y bond.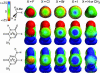

 Cl (green circles), X
Cl (green circles), X Br (red triangles), and X
Br (red triangles), and X I (cyan squares). Both the x and y axes of the plot represent RX···O, with the y axis aligned along the C
I (cyan squares). Both the x and y axes of the plot represent RX···O, with the y axis aligned along the C X bond (180°) and the x axis perpendicular to the C
X bond (180°) and the x axis perpendicular to the C X bond (90°). The shaded region from 90° to 120° indicates the Θ1 angles that were excluded from our data set. (b) Histogram distribution of Θ1 angles. The number of short X···O interactions to chlorine (green), bromine (red), and iodine (cyan) halogen atoms, and their sum (gray) are counted and placed into 5° bins of Θ1 angle and plotted as a 3D histogram. (c) Histogram distribution of Θ2 angles. This plot is similar to b, except the interactions are placed into 10° bins of Θ2. (d) Histogram distribution of the dihedral angle Ψ calculated for short halogen bonds involving the O
X bond (90°). The shaded region from 90° to 120° indicates the Θ1 angles that were excluded from our data set. (b) Histogram distribution of Θ1 angles. The number of short X···O interactions to chlorine (green), bromine (red), and iodine (cyan) halogen atoms, and their sum (gray) are counted and placed into 5° bins of Θ1 angle and plotted as a 3D histogram. (c) Histogram distribution of Θ2 angles. This plot is similar to b, except the interactions are placed into 10° bins of Θ2. (d) Histogram distribution of the dihedral angle Ψ calculated for short halogen bonds involving the O C group of the peptide backbone.
C group of the peptide backbone.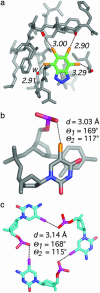
 O group. In addition, one halogen bond to a water molecule (w) is seen (left). (b) Intramolecular halogen bond identified as stabilizing a DNA junction (PDB ID code 1P54) in the 1.9-Å structure of d(CCAGTACbr5UGG) (1). (c) View of the packing interactions involving three short I···O contacts in a unique six-stranded DNA structure (PDB ID code 1UE2; 1.4 Å) of the sequence d(Gi5CGAAAGCT) (i5C, 5-iodocytosine) (28).
O group. In addition, one halogen bond to a water molecule (w) is seen (left). (b) Intramolecular halogen bond identified as stabilizing a DNA junction (PDB ID code 1P54) in the 1.9-Å structure of d(CCAGTACbr5UGG) (1). (c) View of the packing interactions involving three short I···O contacts in a unique six-stranded DNA structure (PDB ID code 1UE2; 1.4 Å) of the sequence d(Gi5CGAAAGCT) (i5C, 5-iodocytosine) (28).References
-
- Hays, F. A., Vargason, J. M. & Ho, P. S. (2003) Biochemistry 42, 9586–9597. - PubMed
-
- Howard, E. I., Sanishvili, R., Cachau, R. E., Mitschler, A., Chevrier, B., Barth, P., Lamour, V., Van Zandt, M., Sibley, E., Bon, C., et al. (2004) Proteins 55, 792–804. - PubMed
-
- Hassel, O. (1972) in Nobel Lectures, Chemistry 1963–1970 (Elsevier, Amsterdam).
-
- Foster, R. (1969) Organic Charge-Tranfer Complexes (Academic, London).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

