Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from differential tissue expression of homospermidine synthase
- PMID: 15557091
- PMCID: PMC535835
- DOI: 10.1104/pp.104.052357
Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from differential tissue expression of homospermidine synthase
Abstract
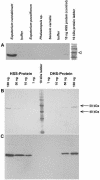
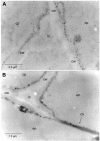
The evolution of pathways within plant secondary metabolism has been studied by using the pyrrolizidine alkaloids (PAs) as a model system. PAs are constitutively produced by plants as a defense against herbivores. The occurrence of PAs is restricted to certain unrelated families within the angiosperms. Homospermidine synthase (HSS), the first specific enzyme in the biosynthesis of the necine base moiety of PAs, was originally recruited from deoxyhypusine synthase, an enzyme involved in the posttranslational activation of the eukaryotic initiation factor 5A. Recently, this gene recruitment has been shown to have occurred several times independently within the angiosperms and even twice within the Asteraceae. Here, we demonstrate that, within these two PA-producing tribes of the Asteraceae, namely Senecioneae and Eupatorieae, HSS is expressed differently despite catalyzing the same step in PA biosynthesis. Within Eupatorium cannabinum, HSS is expressed uniformly in all cells of the root cortex parenchyma, but not within the endodermis and exodermis. Within Senecio vernalis, HSS expression has been previously identified in groups of specialized cells of the endodermis and the adjacent root cortex parenchyma. This expression pattern was confirmed for Senecio jacobaea as well. Furthermore, the expression of HSS in E. cannabinum is dependent on the development of the plant, suggesting a close linkage to plant growth.
Figures



References
-
- Bayer RJ, Starr JR (1998) Tribal phylogeny of the Asteraceae based on two noncoding chloroplast sequences, the trnL intron and trnL/trnF intergenic spacer. Ann Mo Bot Gard 85: 242–256
-
- Böttcher F, Ober D, Hartmann T (1994) Biosynthesis of pyrrolizidine alkaloids: Putrescine and spermidine are essential substrates of enzymatic homospermidine formation. Can J Chem 72: 80–85
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

