Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants
- PMID: 15557094
- PMCID: PMC535854
- DOI: 10.1104/pp.104.052092
Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants
Abstract
Most enzymes in the central pathway of carotenoid biosynthesis in plants have been identified and studied at the molecular level. However, the specificity and role of cis-trans-isomerization of carotenoids, which occurs in vivo during carotene biosynthesis, remained unresolved. We have previously cloned from tomato (Solanum lycopersicum) the CrtISO gene, which encodes a carotene cis-trans-isomerase. To study the biochemical properties of the enzyme, we developed an enzymatic in vitro assay in which a purified tomato CRTISO polypeptide overexpressed in Escherichia coli cells is active in the presence of an E. coli lysate that includes membranes. We show that CRTISO is an authentic carotene isomerase. Its catalytic activity of cis-to-trans isomerization requires redox-active components, suggesting that isomerization is achieved by a reversible redox reaction acting at specific double bonds. Our data demonstrate that CRTISO isomerizes adjacent cis-double bonds at C7 and C9 pairwise into the trans-configuration, but is incapable of isomerizing single cis-double bonds at C9 and C9'. We conclude that CRTISO functions in the carotenoid biosynthesis pathway in parallel with zeta-carotene desaturation, by converting 7,9,9'-tri-cis-neurosporene to 9'-cis-neurosporene and 7'9'-di-cis-lycopene into all-trans-lycopene. These results establish that in plants carotene desaturation to lycopene proceeds via cis-carotene intermediates.
Figures
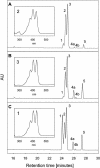
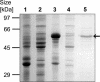


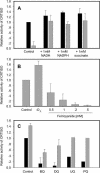
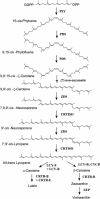
References
-
- Al-Babili S, von Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601–612 - PubMed
-
- Armstrong GA (1997) Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu Rev Microbiol 51: 629–659 - PubMed
-
- Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259: 396–403 - PubMed
-
- Beyer P, Kroncke U, Nievelstein V (1991) On the mechanism of lycopene isomerase cyclase reaction in Narcissus pseudonarcissus L. chromoplasts. J Biol Chem 266: 17072–17078 - PubMed
-
- Beyer P, Mayer M, Kleinig H (1989) Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184: 141–150 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

