A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis
- PMID: 15557347
- PMCID: PMC2211948
- DOI: 10.1084/jem.20040708
A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis
Abstract
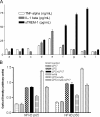
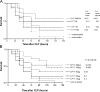
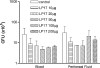
The triggering receptor expressed on myeloid cells (TREM)-1 is a recently discovered receptor expressed on the surface of neutrophils and a subset of monocytes. Engagement of TREM-1 has been reported to trigger the synthesis of proinflammatory cytokines in the presence of microbial products. Previously, we have identified a soluble form of TREM-1 (sTREM-1) and observed significant levels in serum samples from septic shock patients but not controls. Here, we investigated its putative role in the modulation of inflammation during sepsis. We observed that sTREM-1 was secreted by monocytes activated in vitro by LPS and in the serum of animals involved in an experimental model of septic shock. Both in vitro and in vivo, a synthetic peptide mimicking a short highly conserved domain of sTREM-1 appeared to attenuate cytokine production by human monocytes and protect septic animals from hyper-responsiveness and death. This peptide seemed to be efficient not only in preventing but also in down-modulating the deleterious effects of proinflammatory cytokines. These data suggest that in vivo modulation of TREM-1 by sTREM peptide might be a suitable therapeutic tool for the treatment of sepsis.
Figures



References
-
- Aderem, A, and R.J. Ulevitch, R.J. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–786. - PubMed
-
- Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M.T. Ochoa, P.A. Engele, P.A. Sieling, P.F. Barnes, M. Rollinghoff, P.L. Bolcskei, and M. Wagner. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science. 291:1544–1549. - PubMed
-
- Medzhitov, R., and C. Janeway Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338–344. - PubMed
-
- Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. - PubMed
-
- Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

